Articles
- Page Path
- HOME > Epidemiol Health > Volume 41; 2019 > Article
-
Review
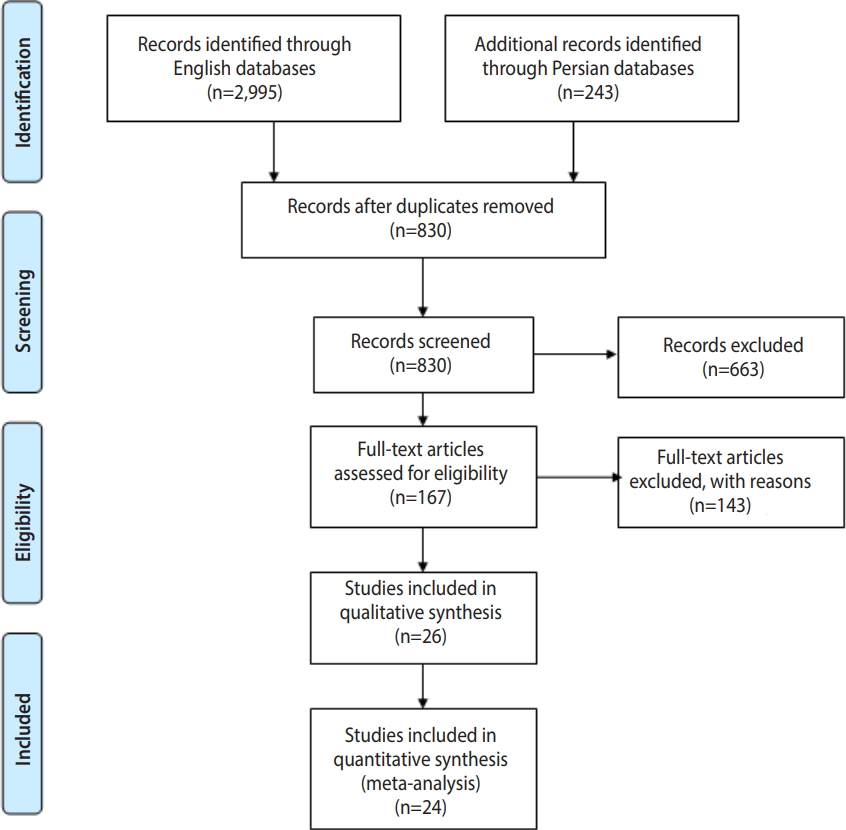
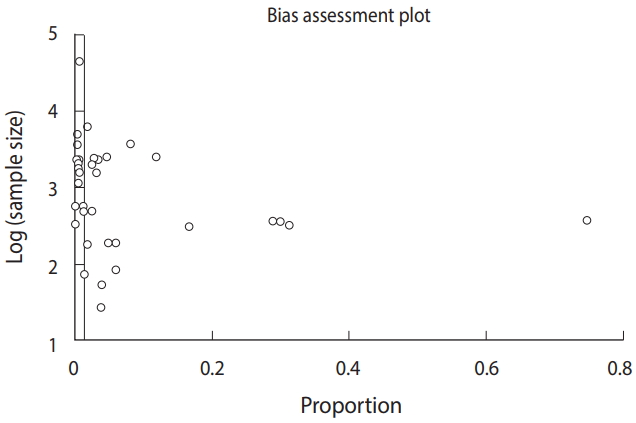
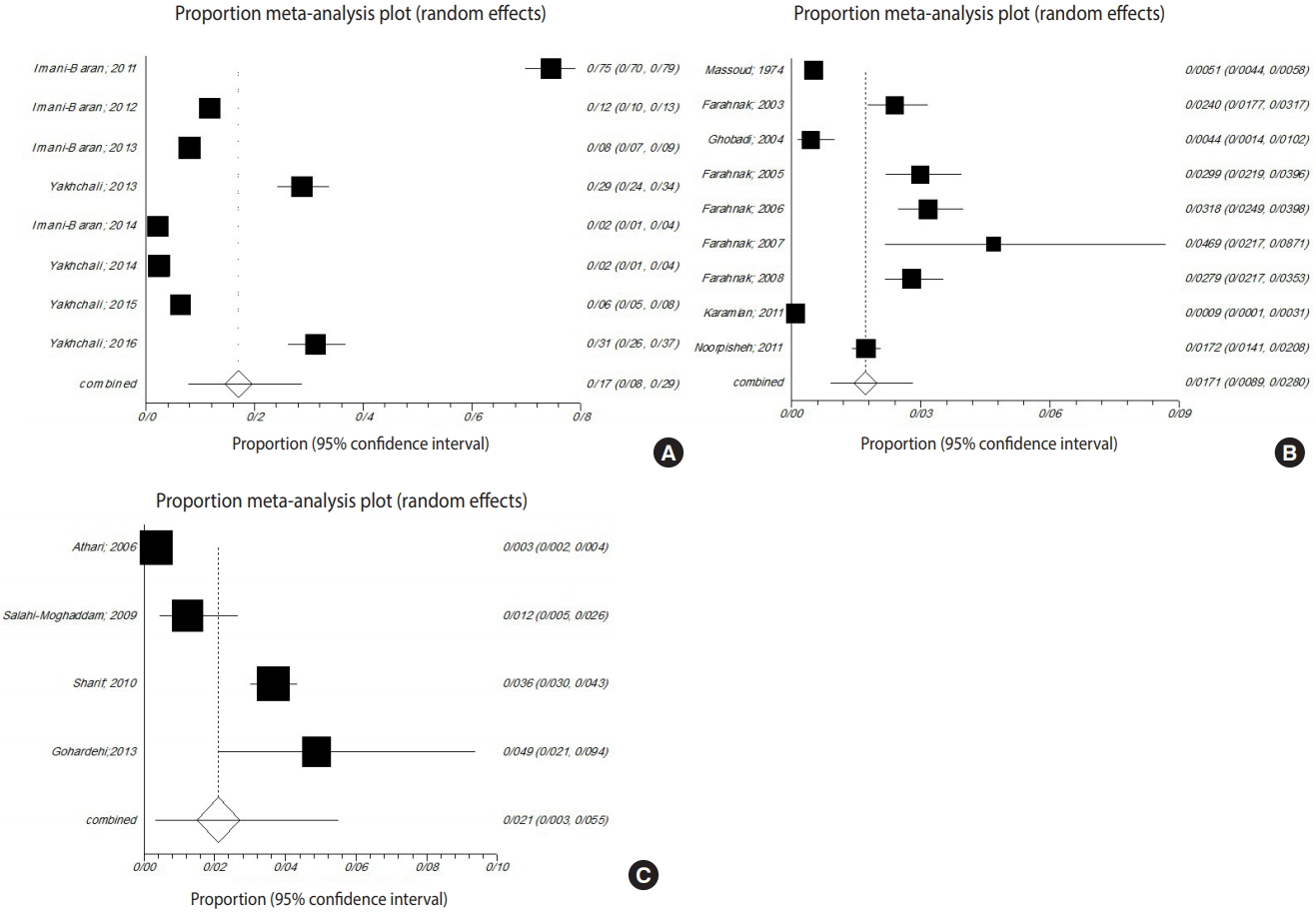
Freshwater snails as the intermediate host of trematodes in Iran: a systematic review -
Samira Dodangeh1
 , Ahmad Daryani2
, Ahmad Daryani2 , Mehdi Sharif2, Shirzad Gholami2
, Mehdi Sharif2, Shirzad Gholami2 , Elham Kialashaki1, Mahmood Moosazadeh3, Shahabeddin Sarvi2
, Elham Kialashaki1, Mahmood Moosazadeh3, Shahabeddin Sarvi2
-
Epidemiol Health 2019;41:e2019001.
DOI: https://doi.org/10.4178/epih.e2019001
Published online: January 7, 2019
1Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran
2Department of Medical Parasitology and Mycology, Toxoplasmosis Research Center, Mazandaran University of Medical Sciences, Sari, Iran
3Health Sciences Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran
- Correspondence: Shahabeddin Sarvi Department of Medical Parasitology and Mycology, Toxoplasmosis Research Center, Mazandaran University of Medical Sciences, P.O. Box 48175-1665, Sari, Iran E-mail: shahabesarvi@yahoo.com
• Received: September 22, 2018 • Accepted: December 16, 2018
©2019, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Isolation and molecular identification of liver fluke cercariae in freshwater snails of Chaharmahal and Bakhtiari province, Iran
Bijan Hosseinpour Aghaei, Nadia Taiefi Nasrabadi, Yaser Pirali Kheirabadi, Seyed Shapoor Reza Shojaei
Molluscan Research.2024; 44(1): 84. CrossRef - Host species of freshwater snails within the same freshwater ecosystem shapes the intestinal microbiome
Zongfu Hu, Qing Tong, Jie Chang, Junzhao Xu, Baiyila Wu, Yongmei Han, Jianhua Yu, Huaxin Niu
Frontiers in Ecology and Evolution.2024;[Epub] CrossRef - Type of cercaria in freshwater snails at Tunggu Pampang Reservoir, Makassar City, Indonesia
Arif Rahman Jabal, Dian Mutiasari, Hairil Akbar, M. Arfah, Marhani Marhani, Rini Rini, Nur Alam Sobak, Anggit Julianingsih Pisu, Agnes Immanuela Toemon, Arini Ratnasari
Russian Journal of Infection and Immunity.2023; 12(4): 765. CrossRef - Infestation with metacercarial stage of Isoparorchis hypselobagri (Billet, 1898) in cage cultured Ompok bimaculatus vis-a-vis host and environmental interaction in a large tropical reservoir
Manoharmayum Shaya Devi, Gunjan Karnatak, Basanta Kumar Das, Asit Kumar Bera, Nilemesh Das, Chayna Jana, Mishal Puthiyottil, Tasso Tayung, Bijay Kumar Behera, Uttam Kumar Sarkar, Yusuf Ali
Aquaculture.2023; 565: 739102. CrossRef - Trematode Cercariae from Lymnaea gedrosiana in the Caspian Sea Littoral in Iran: a one health concern
Aida Vafae Eslahi, Armin Aligolzadeh, Majid Pirestani, Zahra Gharibi, Amir Abdoli, Kareem Hatam-Nahavandi, Behzad Bijani, Milad Badri, Jennifer K. Ketzis
Frontiers in Microbiology.2023;[Epub] CrossRef -
Morphological, molecular, and pathological studies on
Prosthogonimus cuneatus
in Indian peacocks (
Pavo cristatus
)
Asok Kumar Mariappan, Megha Sharma, Karikalan Mathesh, Vivek Srinivas Mouttou, Hiraram, Abhijeet Pawde, Dhama Kuldeep, Saikumar G
Avian Pathology.2023; 52(6): 432. CrossRef - Morphological and molecular characterization of larval trematodes infecting the assassin snail genusAnentomein Thailand
N. Chomchoei, T. Backeljau, B. Segers, C. Wongsawad, P. Butboonchoo, N. Nantarat
Journal of Helminthology.2022;[Epub] CrossRef - Rumen Fluke in Cattle and Buffaloes in Asia: A Review
Nazir Ahmad Tookhy, Md Isa Nur-Mahiza, Rozaihan Mansor, Abd Rahman Yasmin, Nur Indah Ahmad, Noor Hazfalinda Hamzah, Lokman Hakim Idri
Pertanika Journal of Tropical Agricultural Science.2022; 45(3): 781. CrossRef - Molecular and phylogenetic analysis and risk assessment of a trematode parasite, Artyfechinostomum sufrartyfex Lane, 1915 with a new host record from India
Dimple Mandla, Neena Singla, Sukhmanpreet Kaur Brar, Lachhman Das Singla
Biologia.2022; 78(1): 119. CrossRef - Insights on foodborne zoonotic trematodes in freshwater snails in North and Central Vietnam
Phuong Thi Xuan Nguyen, Hien Van Hoang, Huyen Thi Khanh Dinh, Pierre Dorny, Bertrand Losson, Dung Thi Bui, Laetitia Lempereur
Parasitology Research.2021; 120(3): 949. CrossRef - DNA barcoding of Iranian radicine freshwater snails begins to untangle the taxonomy and phylogeography of intermediate hosts of schistosomiasis and fasciolosis from the Middle East and across Central Asia
Ramtin Mirfendereski, Saeid Hashemi, Salome Shirali, Bahar Shemshadi, Scott P. Lawton
Infection, Genetics and Evolution.2021; 89: 104728. CrossRef - Association between human cercarial dermatitis (HCD) and the occurrence of Trichibilarizia in duck and snail in main wetlands from Mazandaran Province, northern Iran
Elham Kia lashaki, Shirzad Gholami, Mahdi Fakhar, Mehdi Karamian, Ahmad Daryani
Parasite Epidemiology and Control.2021; 13: e00211. CrossRef - Essential oils and their components as promising approach for gastropod mollusc control: a review
Mohamed A. Radwan, Amira F. Gad
Journal of Plant Diseases and Protection.2021; 128(4): 923. CrossRef - Annotated and Updated Checklist of Land and Freshwater Molluscs from Asturias (Northern Spain) with Emphasis on Parasite Transmitters and Exotic Species
Omar Sánchez, Jairo Robla, Andrés Arias
Diversity.2021; 13(9): 415. CrossRef - Prevalence of fasciolosis in livestock and humans: A systematic review and meta-analysis in Iran
Shahram Khademvatan, Hamidreza Majidiani, Hamidreza Khalkhali, Ali Taghipour, Negar Asadi, Elham Yousefi
Comparative Immunology, Microbiology and Infectious Diseases.2019; 65: 116. CrossRef - Environmental determinants of distribution of freshwater snails and trematode infection in the Omo Gibe River Basin, southwest Ethiopia
Seid Tiku Mereta, Jemal Bedewi, Delenasaw Yewhalaw, Belayhun Mandefro, Yihun Abdie, Dechassa Tegegne, Wondwosen Birke, Worku Legesse Mulat, Helmut Kloos
Infectious Diseases of Poverty.2019;[Epub] CrossRef

 KSE
KSE

 PubReader
PubReader ePub Link
ePub Link Cite
Cite