Articles
- Page Path
- HOME > Epidemiol Health > Volume 44; 2022 > Article
-
Original Article
Effect of mammography screening on the long-term survival of breast cancer patients: results from the National Cancer Screening Program in Korea -
Xuan Quy Luu1
 , Kyeongmin Lee1
, Kyeongmin Lee1 , Jae Kwan Jun2
, Jae Kwan Jun2 , Mina Suh2
, Mina Suh2 , Kyu-Won Jung2
, Kyu-Won Jung2 , Kui Son Choi1
, Kui Son Choi1
-
Epidemiol Health 2022;44:e2022094.
DOI: https://doi.org/10.4178/epih.e2022094
Published online: October 26, 2022
1Department of Cancer Control and Population Health, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea
2National Cancer Control Institute, National Cancer Center, Goyang, Korea
- Correspondence: Kui Son Choi, Department of Cancer Control and Population Health, Graduate School of Cancer Science and Policy, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang 10408, Korea, E-mail: kschoi@ncc.re.kr
© 2022, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,386 Views
- 173 Download
Abstract
-
OBJECTIVES
- This study investigated the effect of mammography screening on the long-term survival of breast cancer (BC) patients aged 40 years or older according to their screening history and duration since screening.
-
METHODS
- The study cohort was organized from 3 nationwide databases of the Korean National Cancer Screening Program, the Korean Central Cancer Registry, and death certificates. We included 24,387 women diagnosed with invasive BC or ductal carcinoma in situ in 2008 and 2009 and followed up until December 31, 2019. Cox proportional-hazards regression was used to investigate the effect of BC screening on the risk of death.
-
RESULTS
- Overall, 20,916 of 24,387 patients (85.8%) were alive at the end of the follow-up period (median: 10.5 years). The long-term survival rate was significantly lower in the never-screened group (80.3%) than in the screened group (88.9%) (p<0.001). A 35% reduction in the risk of BC death (hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.60 to 0.70) from screening was observed. A subgroup analysis according to the cancer stage showed 62%, 36%, and 24% lower risks of BC death for the localized stage, regional stage, and distant stage, respectively. Women aged 40–49 years received the least benefit from BC screening (HR, 0.71; 95% CI, 0.62 to 0.81).
-
CONCLUSIONS
- Mammography screening was effective in reducing the risk of BC-specific death in Asian women across all cancer stages. However, this effect was relatively small among women in their 40s, suggesting that more detailed and specialized screening strategies are needed for that age group.
- Breast cancer (BC) is the most commonly diagnosed cancer type globally in terms of both the number of new cases and the age-standardized incidence. In 2020, BC accounted for 11.7% of new cancer cases [1]. However, the distribution of BC cases is skewed; approximately more than 80% of BC cases occur in countries with high or very high human development indices. To reduce the high burden of BC, several countries have adopted national cancer screening programs [2], which mostly use mammography as the main screening modality. With the geo-ethnic differences in BC epidemiology, the challenges in implementing the mammography screening program vary across regions [3–5]. In Asia, especially, BC screening has several additional challenges, including screening women in their 40s and the accuracy of mammography in populations with a relatively high prevalence of dense breasts and the relationship thereof to overdiagnosis [3,6]. Therefore, BC screening in Asia should be considered carefully with region-specific evidence [3,4]. However, studies investigating the effectiveness of mammography screening programs in Asia are still limited [7–9].
- In Korea, BC is one of the most common cancer types in women. In contrast with the decreasing overall cancer incidence, BC incidence is steadily increasing. In 2018, the age-standardized rate of BC (57.9 per 100,000) was almost 3 times higher than those of colorectal cancer (20.6 per 100,000) and stomach cancer (18.3 per 100,000) in women [10]. BC incidence peaked at 45–49 years before gradually declining with age [10]. Currently, the Korean National Cancer Screening Program (KNCSP) provides biannual mammography screening examinations for all women aged 40 years or older based on the age-specific pattern of BC incidence in Korea [11]. A previous study evaluated the effect of mammography on BC mortality [12], but it was limited in assessing patients’ long-term survival and the time interval since screening exposure.
- Therefore, this study investigated the effect of mammography on the long-term survival of BC patients aged 40 years or older according to the screening history and time since screening using the KNCSP database. We also assessed the association between mammography screening and survival for ductal carcinoma in situ (DCIS) cases.
INTRODUCTION
- Study materials
- The baseline population comprised 24,454 patients aged 40–79 years who were diagnosed with BC or DCIS between January 1, 2008, and December 31, 2009, and registered in the Korea Central Cancer Registry (KCCR), which covered more than 95% of cancer cases in Korea [10]. Subsequently, we excluded 67 BC patients identified from only death certificates without diagnostic information. Finally, a total of 24,387 women were included in our final analysis.
- Using each resident’s unique 13-digit registration number, we linked our baseline BC patients identified from the KCCR and the KNCSP database for information on the history of mammographic screening between 2002 and 2009. We followed them up from the date of BC diagnosis until the end of 2019, allowing us to determine the long-term survival of the entire study population for at least 10 years. The date and cause of death were ascertained from the death certificates provided by Statistics Korea.
- Measurements
- Information on the date of the primary diagnosis and tumor characteristics was obtained from the KCCR database [10]. We used the International Classification of Diseases, 10th revision (ICD-10) codes [13], and the BC cases were defined as those with the ICD-10 codes C50 and D05. The anatomic sites were grouped into the inner part, the outer part, central portion, and others (nipple and axillary tail of the breast) using the topographical code of the International Classification of Diseases for Oncology, third edition (ICD-O-3) [14]. For the histological subtype, we divided the patients into 4 main groups based on ICD-O-3 morphology codes, as suggested by a previous study: DCIS, ductal carcinoma, lobular carcinoma, and others [15]. The stage at diagnosis was presented as DCIS, localized stage, regional stage, or distant stage according to the summary stage classification in the Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics Review of the National Cancer Institute [16].
- The mammography screening history and the basic socio-demographic information were extracted from the KNCSP database. The mammography screening exposure of BC patients was assessed using screening records from 2002 to 2009, when the KNCSP for BC was introduced [11]. Socioeconomic status was categorized into 3 groups according to the health insurance type: Medical Aid program recipients (people who live under the poverty line and receive livelihood assistance from the government), National Health Insurance beneficiaries with a premium of the 50th percentile or lower, and National Health Insurance beneficiaries with a premium above the 50th percentile.
- Study outcome
- The primary outcome of the study was the long-term survival of BC patients according to their mammography screening history. To ascertain death, all BC patients were followed for at least 10 years or more. The primary outcome of this study was BC-specific mortality. All-cause deaths, including and excluding those from BC as secondary outcomes, were also assessed to adjust for methodological bias, such as misclassification bias and competing risks. Furthermore, the number of person-years of all BC patients was measured from the date of BC diagnosis to the date of death or the end of follow-up, whichever occurred first, corresponding to the end of the observation period (December 31, 2019).
- Statistical analysis
- The baseline characteristics, including socio-demographic information and tumor characteristics, of the screened and never-screened patients were compared using the chi-square test. Kaplan–Meier analysis and the log-rank test were used to compare the survival of subgroups according to screening history.
- We conducted Cox proportional-hazards regression analysis to estimate the hazard ratios (HRs) with 95% confidence intervals (CIs) for investigating the effect of BC screening on the risk of BC death. We assessed the HRs for all-cause death, and non-BC death, to adjust for methodological bias, such as competing risks. All models were adjusted for age, socioeconomic status, and tumor characteristics such as cancer stage, anatomic site, and histological subtype. Additionally, the models were run on several subpopulations stratified by age and cancer stage to investigate variation in the BC screening effects in different groups.
- The net benefit of mammography screening was estimated to address the risk of self-selection bias in a cohort study using a formula suggested by a previous study: net benefit=(HRb−HRa)/HRb×100, where HRb represents the HR for total mortality except BC, and HRa represents the HR for BC-specific mortality [17]. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all the statistical analyses, and p-values of <0.05 denoted statistical significance.
- Ethics statement
- The current retrospective study was approved by the Institutional Review Board of the National Cancer Center, Korea (No. NCCNCS08129). We used de-identified data of a relatively large population from the National Health Insurance Service database, and the requirement for informed consent was waived.
MATERIALS AND METHODS
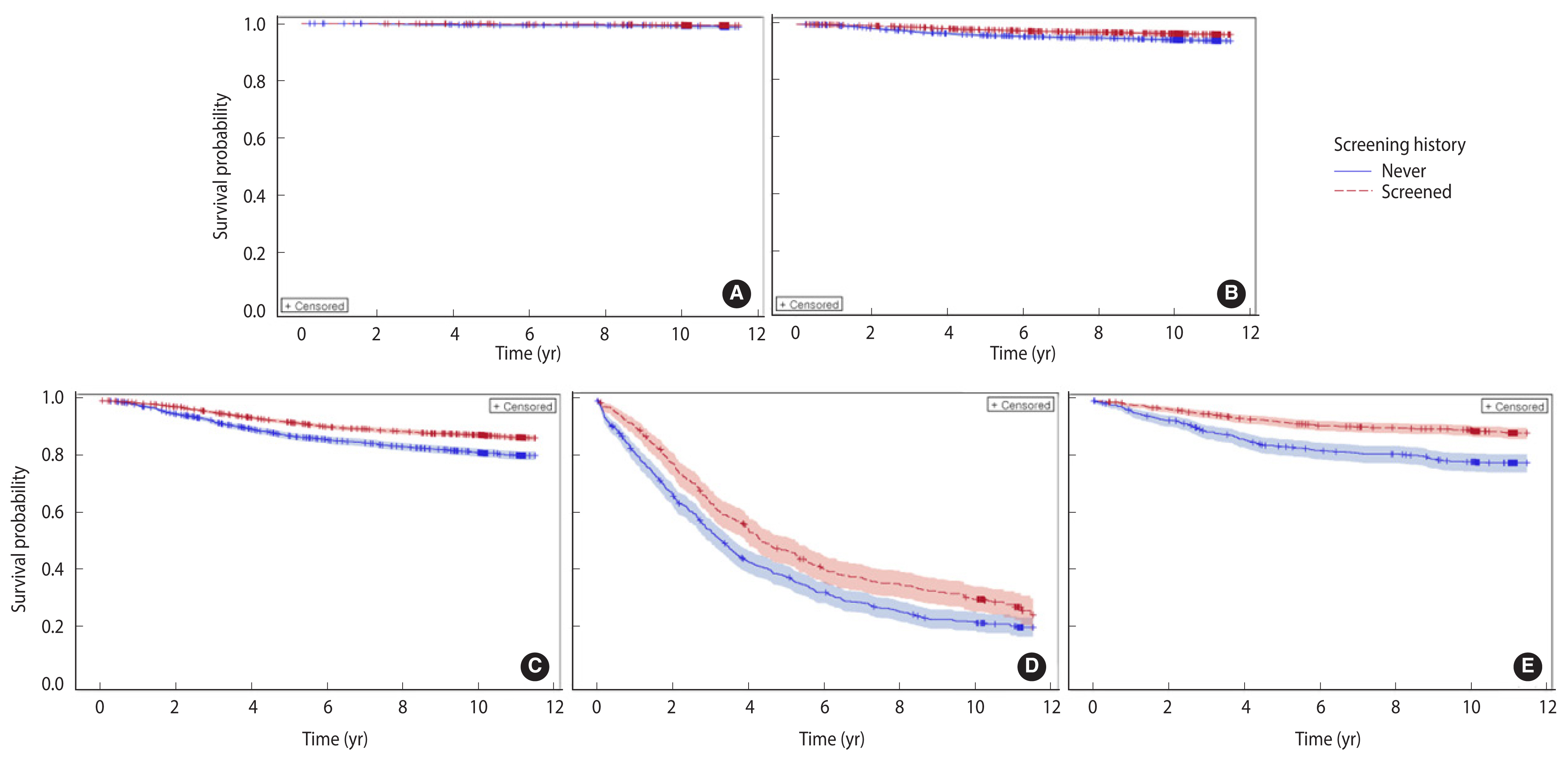
- The baseline characteristics of the BC patients are presented in Table 1. Of the 24,387 BC patients, 8,823 (36.2%) were never screened and 15,564 (63.8%) were screened for BC. Significant differences were found in socio-demographic and tumor characteristics between the never-screened and screened patients. BC involving the inner and outer parts of the breast, DCIS, and localized BC were significantly more prevalent among the screened patients than among the never-screened patients (p<0.001). Of the screened patients, 54.6% underwent screening only once, and 68.5% underwent screening within a year of the date of cancer diagnosis. Overall, 3,471 patients died (14.2%), of whom 2,614 died from BC during the median follow-up duration of 10.5 years (interquartile range, 10.3–11.5). The survival rate was significantly higher among the screened patients (88.9%) than among the never-screened patients (80.3%) (p<0.001). Similar trends of survival were also observed in each cancer stage except DCIS (p=0.28, Figure 1A). The long-term survival was significantly different according to sociodemographic characteristics and screening histories (Supplementary Material 1). The risk of BC-specific death (HRs) increased significantly with age, lower socioeconomic status, and advanced stage at diagnosis.
- The HRs for all-cause death, BC-specific death, and non-BC death according to the screening history are shown in Table 2. For model 1, after adjusting for age and economic status, mammography screening was associated with an approximately 53% lower risk of death from BC (HR, 0.47; 95% CI, 0.44 to 0.51). This figure was 35% for model 2, with additional adjustment of tumor characteristics (HR, 0.65; 95% CI, 0.60 to 70) and the fully adjusted model 3 with invasive cancer only (HR, 0.65; 95% CI, 0.60 to 0.70). A similar decrease was observed for overall death and death from conditions other than BC, with fully adjusted HRs (including invasive cancer only) of 0.68 (95% CI, 0.63 to 0.72) and 0.74 (95% CI, 0.64 to 0.86), respectively.
- The effect of screening on BC mortality varied with age and the stage of BC at diagnosis (Table 3). The risk reduction for BC-specific death was the highest for patients older than 70 years (HR, 0.50; 95% CI, 0.40 to 0.63) who had been screened and the lowest for those in their 40s (HR, 0.71; 95% CI, 0.62 to 0.81). The largest risk reduction for BC-specific death was observed in patients with localized BC who had been screened (HR, 0.58; 95% CI, 0.49 to 0.70). For cases of distant-stage cancer, the risk of death was significantly reduced by approximately 24% (HR, 0.76; 95% CI, 0.66 to 0.89). However, there was no significant difference in BC-specific survival among patients diagnosed with DCIS (HR, 0.62; 95% CI, 0.26 to 1.43; p=0.280). Overall, mammography screening was significantly associated with 42%, 36%, and 24% lower risks of BC death in the localized stage, regional stage, and distant stage subgroups, respectively (Table 3). Given the similar results for all-cause death, BC-specific death, and other causes of death except BC, further subgroup analyses were conducted (Supplementary Material 2).
- Additionally, our results showed similar effects of screening on BC death between people with time intervals since the last screening of less than 1 year (HR, 0.63; 95% CI, 0.57 to 0.69) and 1–2 years (HR, 0.65; 95% CI, 0.56 to 0.76) (Table 4). A 25% lower risk of death from BC was still observed among people who had their last screening more than 2 years earlier (2–3 years: HR, 0.75; 95% CI, 0.62 to 0.91; >3 years: HR, 0.75; 95% CI, 0.62 to 0.91). Furthermore, women who underwent mammography screening more than 3 times before BC diagnosis had a statistically significant 48% reduction in the risk of death from BC.
RESULTS
- Long-term follow-up studies estimating the screening effects of BC patients in Asian women are very limited. The current study reported that the rate of BC-specific deaths was 35% lower among screened BC patients relative to their never-screened counterparts (HR, 0.65; 95% CI, 0.60 to 0.70) based on 10-year follow-up data. This study also provided additional evidence on the stage-specific screening effect; the reductions in the risk of BC death after screening were 42%, 36%, and 26% for patients diagnosed with localized, regional, and distant stages, respectively. A study conducted in Finland involving participants predominantly aged ≥40 years reported that patients with BC detected in a screening program had a 41% lower risk of BC death than those with BC detected outside the screening program after 15 years of follow-up [18]. Similarly, a large-scale study using data from the Swedish Cancer Registry highlighted that 40-year-old to 69-year-old women who underwent mammography had a 41% lower risk of BC death within 10 years [19]. A 57% BC mortality rate reduction in women aged 50–69 years was reported by Kaplan et al. [20] for 5-year survival, and a further reduction was expected at 10 years as the effect of lead-time bias. Kalager et al. [21] reported a 14% reduction in the BC mortality rate after the introduction of a screening program relative to that before the program, which is lower than that reported by our study and other mentioned studies [18,19]. The main reason for this difference is that Kalager et al. [21] divided BC patients into 2 groups (pre-program and post-program) for the analysis, without considering their participation status. Therefore, the effect of mammography on survival may have been underestimated, as some patients in the post-program group did not participate in the screening program.
- The improvement in the overall survival of screened patients could be explained by the stage-shift effect of screening. Screened people have a higher likelihood of cancer being detected at an early or even pre-cancerous stage with a better prognosis, which is well documented in previous studies on mammography screening [7,12,19,21–23] and in our study results. Furthermore, as the basic mechanism of screening is to detect cancer early, even within a stage, screened people have a higher likelihood of cancer being detected at the beginning of this stage. Thus, the early initiation of treatment could have a favorable effect on patient outcomes. Furthermore, the prognosis of patients is also related to the histological characteristics of the tumor. Screening-detected cancer cases are likely to be slowly progressing cancers, which also have a better prognosis than same-stage tumors with more aggressive behavior [24]. Although there is limited research on whether mammography screening improves stage-matched survival, better survival was observed in mammography-examined women among patients with similar tumor sizes, showing consistent results with our findings. Results from a mammographic screening study indicated that improved survival among screened people existed across all subgroup analyses according to tumor size (1–10, 11–20, and 21–50 mm) [18]. Similarly, Joensuu et al. [25] reported an improvement in distant disease-free survival among screened individuals in all tumor size strata, even for patients with large tumors (30 mm or more).
- Mammography screening among women younger than 50 years is controversial, as limited evidence supports its cost-effectiveness among this age group [4,5]. Notwithstanding, some Asian countries have included women in their 40s in their screening guidelines, given the different epidemiological characteristics of Asian populations [3–5]. In the current study, we found that the youngest age group (40–49 years) received the least benefit from mammography screening related to long-term survival (HR, 0.71; 95% CI, 0.62 to 0.81). The higher prevalence of dense breasts in younger women had been addressed by a Korean study; whereas the proportion of women with dense breasts was approximately more than 80% among women in their 40s attending mammography screening, this proportion decreased in the older age groups [26]. The accuracy of mammography screening may be lower for this age group [4]. Therefore, screening for women in their 40s should incorporate age-specific strategies including an appropriate modality and screening interval [4,27–29]. The U.S. Preventive Services Task Force recommendation indicates that screening at the age of 40 years should be based on the individual risk and benefits of mammography [27]. According to the recommendations of the American Cancer Society, 40-year to 44-year old women can undergo mammography screening every year; however, women aged 45–54 and ≥55 years should screen annually and biennially, respectively [28]. The American College of Radiology also recommends annual mammography screening for women starting from 40 years old [29].
- Overdiagnosis by BC screening refers to cases that are detected and diagnosed as BC but never progress to symptomatic and aggressive cancer cases. Therefore, the detection of these cases does not contribute to reducing death and causes harm to patients, such as psychological consequences or unnecessary treatments [30,31]. In the current study, we found a significantly higher proportion of DCIS cases among the screened group (13.9%) than among the never-screened group (10.0%) but no significant reduction of the long-term BC mortality rate attributable to screening. While this might be associated with the successful treatment of early cancer, there could also be several cases of overdiagnosis in this group. It is well known that DCIS patients have an excellent prognosis, with a survival rate of more than 95% during the long-term follow-up [31], which is consistent with the long-term survival rate of 96.4% in our study. Moreover, a study from SEER indicated that low-grade DCIS patients in surgery and non-surgery groups had the same survival rates [32]. In contrast, women diagnosed with DCIS are not well aware of their actual risk of developing invasive cancer, which worsens their screening-related psychological issues [31]. Given the increasing trend of DCIS associated with the widespread implementation of screening, it is essential to improve the understanding of DCIS in the population and a shared decision-making strategy for DCIS treatment between patients and physicians to minimize the risk of overdiagnosis and overtreatment.
- Mammography screening has challenges that should be taken into careful consideration when evaluating its effectiveness. Firstly, the cancer cases detected by screening appear to have demonstrated longer survival, which is attributable to the earlier diagnosis (i.e., lead-time bias). In our cohort, the effect of lead-time bias may have been minimal due to the follow-up duration of at least 10 years for all patients. In addition, there is also the risk of selection bias stemming from the different baseline characteristics of people who participate in screening and those who do not. To partially control this in our study, we used several models with different population levels of adjustment of socioeconomic and tumor characteristics. Since BC screening is universally provided and the lifetime screening rate is more than 80% [11], the selection bias would be small. Furthermore, we also used a formula suggested by a previous study to address self-selection bias in a cohort study [17], and the calculated net benefit from mammography screening in our study was 12.2% for invasive cancer only (Table 2) and 17.6% after excluding DCIS and distant cancer cases (Supplementary Material 3).
- Nevertheless, our study had several limitations. First, our study could not combine opportunistic screening information with the screening history. People who did not receive screening through the KNCSP may have already undergone opportunistic screening, which is often performed using ultrasonography as a screening test. Therefore, the effect of BC screening through the KNCSP could be underestimated. Second, our study only assessed the extent of BC based on the summary stage classification by the SEER Cancer Statistics Review of the National Cancer Institute [16], which had limitations in investigating the stage-specific effect. Lastly, as our study covered all BC patients diagnosed in 2008 and 2009 in Korea, it was not possible to include information about treatment, which is a strong predictor of patient prognosis, in our analysis. However, we believe that the treatments received by people in the screened and never-screened groups would have been similar at the same stage, as all Korean residents are enrolled in the National Health Insurance Service. Despite these limitations, this is one of the first studies evaluating the effect of mammography on the long-term survival of BC patients in Asia, to the best of our knowledge. Our study linked information at the individual level from 3 national databases with nearly complete data on screening, cancer, and death information. This makes our findings relatively generalizable to the entire population of the country.
- In conclusion, our study found a significant improvement in the survival of BC patients after mammography screening during a long-term follow-up of 10 years. Our results also indicated a smaller effect of mammography among women in their 40s, who require more detailed and specialized screening strategies. Future studies should have appropriate designs to directly address the overdiagnosis by mammography screening, especially among DCIS patients.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Material 1.
Supplementary Material 2.
Supplementary Material 3.
ACKNOWLEDGEMENTS
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
-
FUNDING
This study was funded by a Grant-in-Aid for Cancer Research and Control from the National Cancer Center, Korea (#2210772).
-
AUTHOR CONTRIBUTIONS
Conceptualization: Choi KS, Luu XQ. Data curation: Lee K, Luu XQ. Formal analysis: Lee K, Luu XQ. Funding acquisition: Choi KS. Methodology: Luu XQ, Jun JK, Suh M, Choi KS. Project administration: Lee K. Writing – original draft: Luu XQ. Writing – review & editing: Luu XQ, Lee K, Jun JK, Jung KW, Suh M, Choi KS.
NOTES
| Variables | Death | Person-years | Death rate per 1,000 | Crude | Model 11 | Model 22 | Model 33 |
|---|---|---|---|---|---|---|---|
| All-cause death | |||||||
| Never-screened | 1,742 | 84,910.2 | 20.5 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Screened | 1,729 | 158,590.1 | 10.9 | 0.53 (0.50, 0.57) | 0.52 (0.48, 0.55) | 0.67 (0.62, 0.71) | 0.68 (0.63, 0.72) |
|
|
|||||||
| BC death | |||||||
| Never-screened | 1,371 | 84,910.2 | 16.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Screened | 1,243 | 158,590.1 | 7.8 | 0.49 (0.45, 0.53) | 0.47 (0.44, 0.51) | 0.65 (0.60, 0.70) | 0.65 (0.60, 0.70) |
|
|
|||||||
| Non-BC death | |||||||
| Never-screened | 371 | 84,910.2 | 4.4 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Screened | 486 | 158,590.1 | 3.1 | 0.70 (0.61, 0.80) | 0.67 (0.59, 0.77) | 0.69 (0.60, 0.79) | 0.74 (0.64, 0.86) |
Values are presented as hazard ratio (95% confidence interval).
BC, breast cancer.
1 Adjusted for age and socioeconomic status.
2 Adjusted for age, socioeconomic status, stage, histological subtype, and anatomic site.
3 Adjusted for age, socioeconomic status, stage, anatomic site, and histological subtype (invasive cancer only, n=21,341).
| Variables | No. of BC deaths | Person-years | Death rate per 1,000 | Fully adjusted HR (95% CI)1 |
|---|---|---|---|---|
| Stage at diagnosis | ||||
| DCIS | ||||
| Never-screened | 9 | 9,417.4 | 0.96 | 1.00 (reference) |
| Screened | 14 | 23,173.5 | 0.60 | 0.62 (0.26, 1.43) |
| Localized | ||||
| Never-screened | 220 | 39,763.1 | 5.53 | 1.00 (reference) |
| Screened | 266 | 79,090.9 | 3.36 | 0.58 (0.49, 0.70) |
| Regional | ||||
| Never-screened | 529 | 26,923.9 | 19.65 | 1.00 (reference) |
| Screened | 551 | 43,473.4 | 12.67 | 0.64 (0.57, 0.73) |
| Distant | ||||
| Never-screened | 474 | 2,796.0 | 169.53 | 1.00 (reference) |
| Screened | 297 | 2,318.0 | 128.13 | 0.76 (0.66, 0.89) |
| Unknown | ||||
| Never-screened | 139 | 6,009.9 | 23.13 | 1.00 (reference) |
| Screened | 115 | 10,534.4 | 10.92 | 0.48 (0.37, 0.62) |
|
|
||||
| Age at diagnosis (yr)2 | ||||
| 40–49 | ||||
| Never-screened | 504 | 36,728.3 | 12.11 | 1.00 (reference) |
| Screened | 406 | 53,949.7 | 6.44 | 0.71 (0.62, 0.81) |
| 50–59 | ||||
| Never-screened | 442 | 23,675.6 | 16.74 | 1.00 (reference) |
| Screened | 454 | 49,914.5 | 7.90 | 0.65 (0.57, 0.74) |
| 60–69 | ||||
| Never-screened | 212 | 9,933.9 | 19.78 | 1.00 (reference) |
| Screened | 252 | 23,777.7 | 9.21 | 0.61 (0.51, 0.74) |
| ≥70 | ||||
| Never-screened | 204 | 5,155.2 | 36.05 | 1.00 (reference) |
| Screened | 117 | 7,774.7 | 13.37 | 0.50 (0.40, 0.63) |
| Variables | No. of BC deaths | Person-years | Death rate per 1,000 | Fully adjusted HR (95% CI)1 |
|---|---|---|---|---|
| Screening frequency (times) | ||||
| Never screened | 1,362 | 75,492.9 | 18.04 | 1.00 (reference) |
| 1 | 739 | 74,426.1 | 9.93 | 0.70 (0.64, 0.77) |
| 2 | 333 | 38,938.3 | 8.55 | 0.62 (0.55, 0.70) |
| ≥3 | 157 | 22,052.3 | 7.12 | 0.52 (0.44, 0.62) |
| p-value for trend | <0.001 | |||
|
|
||||
| Time interval since screening (mo) | ||||
| Never screened | 1,362 | 75,492.9 | 18.04 | 1.00 (reference) |
| ≥36 | 108 | 10,498.5 | 10.29 | 0.75 (0.62, 0.92) |
| 24–35 | 111 | 9,694.3 | 11.45 | 0.75 (0.62, 0.91) |
| 12–23 | 206 | 22,208.6 | 9.28 | 0.65 (0.56, 0.75) |
| ≤11 | 804 | 93,015.3 | 8.64 | 0.63 (0.57, 0.69) |
| p-value for trend | <0.001 | |||
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-249.ArticlePubMedPDF
- 2. rganization for Economic Cooperation and Development (OECD). Screening, survival and mortality for breast cancer. Health at a Glance 2017: OECD indicators. Paris: OECD Publishing; 2017.Article
- 3. Bhoo-Pathy N, Yip CH, Hartman M, Uiterwaal CS, Devi BC, Peeters PH, et al. Breast cancer research in Asia: adopt or adapt Western knowledge? Eur J Cancer 2013;49:703-709.ArticlePubMed
- 4. Green M, Raina V. Epidemiology, screening and diagnosis of breast cancer in the Asia–Pacific region: current perspectives and important considerations. Asia Pac J Clin Oncol 2008;4(Suppl 3):S5-S13.Article
- 5. Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-cancer screening--viewpoint of the IARC Working Group. N Engl J Med 2015;372:2353-2358.ArticlePubMed
- 6. Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol 2001;30:959-965.ArticlePubMed
- 7. Choi KS, Yoon M, Song SH, Suh M, Park B, Jung KW, et al. Effect of mammography screening on stage at breast cancer diagnosis: results from the Korea National Cancer Screening Program. Sci Rep 2018;8:8882.ArticlePubMedPMCPDF
- 8. Huang Y, Dai H, Song F, Li H, Yan Y, Yang Z, et al. Preliminary effectiveness of breast cancer screening among 1.22 million Chinese females and different cancer patterns between urban and rural women. Sci Rep 2016;6:39459.ArticlePubMedPMCPDF
- 9. Yen AM, Tsau HS, Fann JC, Chen SL, Chiu SY, Lee YC, et al. Population-based breast cancer screening with risk-based and universal mammography screening compared with clinical breast examination: a propensity score analysis of 1 429 890 Taiwanese women. JAMA Oncol 2016;2:915-921.ArticlePubMed
- 10. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat 2021;53:301-315.ArticlePubMedPMCPDF
- 11. Hong S, Lee YY, Lee J, Kim Y, Choi KS, Jun JK, et al. Trends in cancer screening rates among korean men and women: results of the Korean National Cancer Screening Survey, 2004–2018. Cancer Res Treat 2021;53:330-338.ArticlePubMedPMCPDF
- 12. Choi E, Jun JK, Suh M, Jung KW, Park B, Lee K, et al. Effectiveness of the Korean National Cancer Screening Program in reducing breast cancer mortality. NPJ Breast Cancer 2021;7:83.ArticlePubMedPMCPDF
- 13. World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision. 2nd ed. 2004 [cited 2022 Jul 4]. Available from: https://apps.who.int/iris/handle/10665/42980 .
- 14. World Health Organization. International classification of diseases for oncology (ICD-O). 3rd ed. 1st revision. 2013 [cited 2022 Jul 4]. Available from: https://apps.who.int/iris/handle/10665/96612 .
- 15. Li CI, Moe RE, Daling JR. Risk of mortality by histologic type of breast cancer among women aged 50 to 79 years. Arch Intern Med 2003;163:2149-2153.ArticlePubMed
- 16. Young JL Jr, Roffers SD, Ries LA, Fritz AG, Hurlbut AA. SEER summary staging manual - 2000: codes and coding instructions. 2001 [cited 2022 Jul 4]. Available from: https://seer.cancer.gov/tools/ssm/ssm2000/SSSM2000-122012.pdf .
- 17. Mizoue T, Yoshimura T, Tokui N, Hoshiyama Y, Yatsuya H, Sakata K, et al. Prospective study of screening for stomach cancer in Japan. Int J Cancer 2003;106:103-107.ArticlePubMed
- 18. Lehtimäki T, Lundin M, Linder N, Sihto H, Holli K, Turpeenniemi-Hujanen T, et al. Long-term prognosis of breast cancer detected by mammography screening or other methods. Breast Cancer Res 2011;13:R134.ArticlePubMedPMCPDF
- 19. Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, et al. Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer 2020;126:2971-2979.ArticlePubMedPDF
- 20. Kaplan HG, Malmgren JA, Atwood MK, Calip GS. Effect of treatment and mammography detection on breast cancer survival over time: 1990–2007. Cancer 2015;121:2553-2561.ArticlePubMedPMC
- 21. Kalager M, Haldorsen T, Bretthauer M, Hoff G, Thoresen SO, Adami HO. Improved breast cancer survival following introduction of an organized mammography screening program among both screened and unscreened women: a population-based cohort study. Breast Cancer Res 2009;11:R44.ArticlePubMedPMCPDF
- 22. Bordás P, Jonsson H, Nyström L, Lenner P. Survival from invasive breast cancer among interval cases in the mammography screening programmes of northern Sweden. Breast 2007;16:47-54.ArticlePubMed
- 23. Klemi PJ, Parvinen I, Pylkkänen L, Kauhava L, Immonen-Räihä P, Räsänen O, et al. Significant improvement in breast cancer survival through population-based mammography screening. Breast 2003;12:308-313.ArticlePubMed
- 24. Tabár L, Duffy SW, Vitak B, Chen HH, Prevost TC. The natural history of breast carcinoma: what have we learned from screening? Cancer 1999;86:449-462.ArticlePubMed
- 25. Joensuu H, Lehtimäki T, Holli K, Elomaa L, Turpeenniemi-Hujanen T, Kataja V, et al. Risk for distant recurrence of breast cancer detected by mammography screening or other methods. JAMA 2004;292:1064-1073.ArticlePubMed
- 26. Bae JM, Shin SY, Kim EH, Kim YN, Nam CM. Distribution of dense breasts using screening mammography in Korean women: a retrospective observational study. Epidemiol Health 2014;36:e2014027.PubMedPMC
- 27. Siu AL; U. S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2016;164:279-296.ArticlePubMed
- 28. Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015;314:1599-1614.ArticlePubMedPMC
- 29. Monticciolo DL, Newell MS, Hendrick RE, Helvie MA, Moy L, Monsees B, et al. Breast cancer screening for average-risk women: recommendations from the ACR commission on breast imaging. J Am Coll Radiol 2017;14:1137-1143.ArticlePubMed
- 30. Helvie MA. Perspectives on the overdiagnosis of breast cancer associated with mammographic screening. J Breast Imaging 2019;1:278-282.ArticlePubMedPDF
- 31. van Seijen M, Lips EH, Thompson AM, Nik-Zainal S, Futreal A, Hwang ES, et al. Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer 2019;121:285-292.ArticlePubMedPMCPDF
- 32. Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 2015;1:888-896.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Figure
- Related articles
-
- Socioeconomic inequality in organized and opportunistic screening for colorectal cancer: results from the Korean National Cancer Screening Survey, 2009-2021
- Trends in breast cancer screening rates among Korean women: results from the Korean National Cancer Screening Survey, 2005-2020
- Effect of Pap smears on the long-term survival of cervical cancer patients: a nationwide population-based cohort study in Korea
- Income-based disparities in the risk of distant-stage cervical cancer and 5-year mortality after the introduction of a National Cancer Screening Program in Korea

 KSE
KSE

 PubReader
PubReader ePub Link
ePub Link Cite
Cite



