Articles
- Page Path
- HOME > Epidemiol Health > Volume 44; 2022 > Article
-
COVID-19
Original Article
Seroprevalence of SARS-CoV-2 antibodies in the community based on participants in the 2020 Korea National Health and Nutrition Examination Survey -
Ah-Ra Kim1
 , Dohsik Minn2
, Dohsik Minn2 , Su Hwan Kim1
, Su Hwan Kim1 , Hyeon Nam Do1
, Hyeon Nam Do1 , Byoungguk Kim1
, Byoungguk Kim1 , Young Sill Choi3
, Young Sill Choi3 , Dong-Hyun Kim4
, Dong-Hyun Kim4 , Eun-Jee Oh5
, Eun-Jee Oh5 , Kyungwon Oh7
, Kyungwon Oh7 , Donghyok Kwon7
, Donghyok Kwon7 , Jun-Wook Kwon8
, Jun-Wook Kwon8 , Sung Soon Kim9
, Sung Soon Kim9 , June-Woo Lee1
, June-Woo Lee1
-
Epidemiol Health 2022;44:e2022028.
DOI: https://doi.org/10.4178/epih.e2022028
Published online: February 21, 2022
1Division of Vaccine Clinical Research, Center for Vaccine Research, National Institute of Infectious Diseases, Cheongju, Korea
2Medical Director for Diagnostic Immunology, Seegene Medical Foundation, Seoul, Korea
3Division of Pathogen Resource Management, Center for Vaccine Research, National Institute of Infectious Diseases, Cheongju, Korea
4Department of Social and Preventive Medicine, Hallym University College of Medicine, Chuncheon, Korea
5Department of Laboratory Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
6Division of Health and Nutrition Survey and Analysis, Bureau of Chronic Disease Prevention and Control, Korea Disease Control and Prevention Agency, Cheongju, Korea
7Division of Public Health Emergency Responses Research, Korea Disease Control and Prevention Agency, Cheongju, Korea
8National Institute of Health (NIH), Korea Disease Control and Prevention Agency, Cheongju, Korea
9Center for Vaccine Research, National Institute of Infectious Diseases, Korea Disease Control and Prevention Agency, Cheongju, Korea
- Correspondence: Sung Soon Kim Center for Vaccine Research, National Institute of Infectious Diseases, Korea Disease Control and Prevention Agency, 212 Osongsaengmyoung 2-ro, Heungdeok-gu, Cheongju 28160, Korea E-mail: sungskim63@korea.kr
- Co-correspondence: June-Woo Lee Division of Vaccine Clinical Research, Center for Vaccine Research, National Institute of Infectious Diseases, 212 Osongsaengmyoung 2-ro, Heungdeok-gu, Cheongju 28160, Korea E-mail: junewoo1213@korea.kr
©2022, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
OBJECTIVES
- The Korea National Health and Nutrition Examination Survey (KNHANES) is a nationwide cross-sectional surveillance system that assesses the health and nutritional status of the Korean population. To evaluate the occurrence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the community, we investigated the prevalence of anti-SARS-CoV-2 antibodies in the sera of KNHANES participants.
-
METHODS
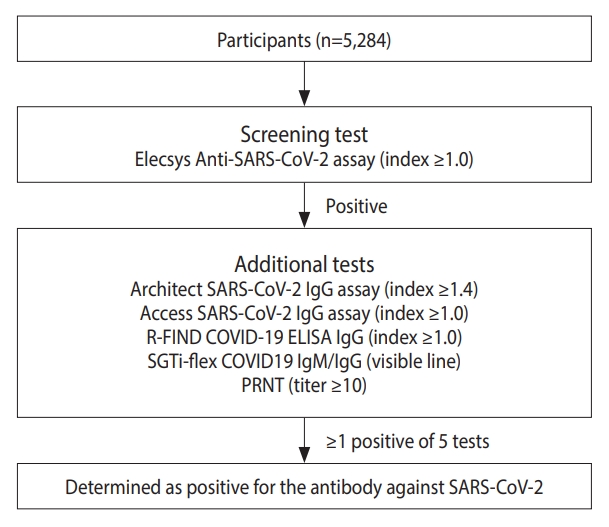
- Subjects were recruited between April 24 and December 12, 2020. In total, 5,284 subjects aged 10-90 years from 17 regions participated. SARS-CoV-2 antibodies were screened using the Elecsys Anti-SARS-CoV-2 assay. Positive samples were verified using 4 different SARS-CoV-2 antibody assays and the plaque reduction neutralizing test. The final seropositivity criteria were a positive screening test and at least 1 positive result from the 5 additional tests.
-
RESULTS
- Almost half (49.2%; 2,600/5,284) of participants were from metropolitan areas, 48.9% were middle-aged (40-69 years), and 20.5% were in their 20s or younger. The seropositivity rate was 0.09% (5/5,284). Three of the 5 antibody-positive subjects had a history of infection, of whom 2 were infected abroad and 1 was infected in a local cluster outbreak.
-
CONCLUSIONS
- The low SARS-CoV-2 antibody seroprevalence in Korea indicates that there have been few coronavirus disease 2019 (COVID-19) cases due to successful COVID-19 management measures (e.g., diagnostic tests for overseas arrivals, national social distancing, and strict quarantine measures). Moreover, asymptomatic infections were uncommon due to active polymerase chain reaction testing. However, hidden infections may exist in the community, requiring the continuation of quarantine and vaccination measures.
- Coronavirus disease 2019 (COVID-19) first emerged in Wuhan, China in December 2019, and then spread rapidly across the world [1]. The World Health Organization (WHO) declared COVID-19 as a global pandemic on March 11, 2020 [2]. By December 31, 2020, a total of 82,629,416 confirmed cases, including 1,813,193 deaths, were reported worldwide [3]. Korea reported the first confirmed case on January 20, 2020, and by December 31, 2020, 60,740 confirmed cases and 900 deaths had occurred [4-6]. In 2020, there were 3 large COVID-19 waves in Korea. The first outbreak occurred in Daegu in February 2020, the second outbreak in metropolitan areas, while the third outbreak occurred sporadically across the nation in December 2020 [6,7].
- Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which causes COVID-19, can spread easily in a population. Although the infection manifests with mild to severe symptoms, there is increasing evidence of asymptomatic infections that can result in viral transmission [8]. Hence, the detection of asymptomatic individuals at an early stage is critical for the prevention and control of COVID-19 spread. Korea reported 3 asymptomatic infections out of 28 confirmed cases (10.7%) in the early stages of an outbreak [9]. Moreover, 62.0% of COVID-19 patients (6,350/10,237) were asymptomatic from January 24 to April 9, 2020, and the asymptomatic positivity rate increased upon pre-emptive testing [10]. Therefore, a study was planned to estimate asymptomatic infections in the community and to assess the seroprevalence of SARS-CoV-2 antibodies in the general population.
- The Korea National Health and Nutrition Examination Survey (KNHANES) is a nationwide cross-sectional surveillance system that assesses the health and nutritional status of Koreans through health interviews, examinations, and nutrition surveys. The KNHANES is conducted every year, and includes 10,000 individuals aged 1 year and above [11]. However, the blood and urine samples for the health examination are collected from participants aged 10 years and above. In the present study, we evaluated the efficiency of the existing COVID-19 prevention and control policies by studying the seroprevalence of SARS-CoV-2 antibodies using the KNHANES samples.
INTRODUCTION
- Study subjects
- This study was conducted on the residual serum samples of KNHANES participants recruited between April 24 and December 12, 2020. At that time, the COVID-19 vaccine had not been introduced in Korea. A total of 5,284 participants aged 10-90 years were recruited from 17 regions, which were classified as provinces and metropolitan areas. The samples were collected 3 times, at 2-month intervals: (1) first collection period (April 24 to June 19) - 1,555 samples, (2) second collection period (June 24 to August 13) - 1,440 samples, (3) third collection period (August 14 to October 31) - 1,379 samples, and (4) fourth collection period (November 1 to December 12) - 910 samples.
- Detection of anti-SARS-CoV-2 antibodies
- SARS-CoV-2 antibodies were screened using the Elecsys Anti-SARS-CoV-2 assay on the Cobas e801 analyzer (Roche Diagnostics, Mannheim, Germany). This kit detects antibodies against the nucleocapsid protein of SARS-CoV-2 with a specificity and sensitivity of 100% and 99.8%, respectively [12]. Positive samples were further validated using 5 additional assays that detected various antigens using different detection principles to improve the specificity of the test results: the Architect SARS-CoV-2 IgG assay (Abbott Laboratories, Chicago, IL, USA), Access SARS-CoV-2 IgG assay (Beckman Coulter, Pasadena, CA, USA), R-FIND COVID-19 ELISA (SG Medical, Inc., Seoul, Korea), SGTi-flex COVID19 IgM/IgG (Sugentech, Daejeon, Korea) rapid kits and the plaque reduction neutralizing test (PRNT) for neutralizing antibodies. All test kit assays were performed according to the manufacturer’s instructions.
- A sample was ultimately considered positive for SARS-CoV-2 antibodies if both the screening test and at least 1 of the 5 additional tests were positive (Figure 1). All seropositive subjects were checked for their infection and travel history during the pandemic, along with their previous polymerase chain reaction test results.
- Ethics statement
- This study was approved by the Institutional Review Board of Korea Disease Control and Prevention Agency (IRB No. 2018-01-03-3C-A).
MATERIALS AND METHODS
- The age, sex, and regional distribution of the 5,284 participants are shown in Table 1. The highest number of participants were from the age group of 70 and above (19.0%), followed by those in their 60s and 50s (17.4 and 16.5%, respectively). In contrast, participants in the age ranges of 10-19 years and 20-29 years constituted the smallest groups (9.4 and 11.1%, respectively). The percentages of females and males were 54.8% and 45.2%, respectively. The distribution of study subjects was designed by considering the proportion of population composition by provinces and metropolitan cities. The metropolitan area (Gyeonggi Province and Incheon) had the highest percentage of participants (30.6%), followed by the city of Seoul and Gyeongsang Province (18.6 and 13.0%, respectively). Daegu, the location of the first cluster outbreak, had 3.2% participants.
- Thirteen seropositive cases were detected in the screening test, which decreased to 5 after the results of the additional assays were taken into account (Table 2). The assay with the highest similarity to the screening test in performance was the Architect SARS-CoV-2 IgG assay, followed by PRNT. Only 1 sample was positive in the screening test and only 1 additional test, while the rest were positive in 2 or more additional tests.
- The number of positive samples in the second, third, and fourth collection periods was 1 (0.07%), 3 (0.22%), and 1 (0.11%), respectively. Among the five seropositive subjects, only 3 (0.06%) had a known history of infection. The confirmed epidemiological information for the 5 seropositive samples is shown in Table 3. Two positive participants were from Seoul, 1 from Gangwon Province, and 2 from Gyeonggi Province. The 2 positive patients from Gyeonggi Province contracted the disease abroad, while the patient in Seoul was infected in a local outbreak. The duration of the antibodies in the seropositive subjects with a history of infection was 12 weeks to 24 weeks.
RESULTS
- COVID-19 emerged in December 2019 and spread rapidly worldwide. By the end of December 2020, the United States had the highest number of confirmed cases (19,513,331), while the United Kingdom and Japan had 2,532,601 and 230,304 confirmed cases, respectively [3]. By that time, Korea had reported 60,740 confirmed cases, which was substantially lower than the number in other countries [4,5].
- Population-based seroprevalence studies of SARS-CoV-2 have been conducted in several countries. In the United States, the seroprevalence of SARS-CoV-2 antibodies using rapid kits was 1.5% (n=3,330) and 4.3% (n=863) in Santa Clara County and California, respectively [13,14]. In Indiana, where antibodies were tested using chemiluminescence immunoassay (CLIA), the seroprevalence was reported as 2.8% (n=3,658) [15]. More recently, the United States Centers for Disease Control and Prevention conducted a seroprevalence survey comparing the results of 5 serosurveys using random sampling of the general population (n=22,118), and the results were estimated at 14.3% [16]. In England, the COVID-19 seropositivity rate, as reported by Public Health England, was 5.5% using the enzyme-linked immunosorbent assay (ELISA) method (n=7,857) [17]. Turning to the neighboring countries of Korea, China showed a seroprevalence of 1.68% and 0.38% from Wuhan and other places, respectively [18]. In Japan, the Utsunomiya COVID-19 seROprevalence Neighborhood Association study conducted in the city of Utsunomiya reported 0.4% seroprevalence [19]. A nationwide seroprevalence study of SARS-CoV-2 antibodies in Korea was recently published. This study was conducted between late September and early December 2020, and the seroprevalence was 0.39% (16/4,085) [20]. However, our study makes a meaningful contribution in that it analyzed seroprevalence using samples from KNHANES participants obtained over a long period (from April to December 2020). The KNHANES samples were representative of the Korean population, due to the sample design based on the age-specific and region-specific Korean populations.
- In this study, the seropositivity of SARS-CoV-2 antibodies in the general population was 0.09% (95% confidence interval, 0.09 to 0.10) (Table 2). This is a very low seroprevalence of SARS-CoV-2 antibodies compared to other countries. This result indicates that the initial testing program was successful in detecting early-stage infections, and the level of hidden infections was maintained at a significantly low level through quarantine control.
- The U.S. Food and Drug Administration recommends not using antibody testing for COVID-19 diagnosis because of high false-positive and false-negative rates [21]. Hence, we validated our initial screening test with 5 additional tests to reduce the chances of false-positive or false-negative results. Each additional test used different antigens, methods, and antibody isotypes. The Roche, Abbott, and SG Medical reagents use the nucleocapsid protein as an antigen, the Beckman reagent uses the spike protein, and the Sugentech reagent uses both. As a measurement method, Roche uses electrochemiluminescence immunoassay, Abbott uses chemiluminescence microparticle immunoassay, Beckman uses CLIA, SG Medical uses ELISA, and Sugentech uses lateral flow. The antibody isotypes detected were also diverse, including total antibodies, immunoglobulin (Ig)G, and IgM.
- Only 1 case was positive for the antibody in only 2 tests, while the other 4 cases showed positive results in 3 or more tests. Furthermore, neutralizing antibodies against the wild virus were also tested. Eight out of the 13 cases were positive in the screening test only, and since there was a high possibility of these being false-positives, they were excluded from the final positive results (Table 2). All 4 cases that showed positive results in 2 or more binding antibody tests were also positive for neutralizing antibodies. There was no correlation between the binding and neutralizing antibodies, but this relationship was difficult to judge because of the small number of relevant cases.
- The KNHANES surveys more than 10,000 people each year. Therefore, we used the KNHANES samples to obtain a large sample size, thereby providing as true a picture of the general population as possible. However, this study has some limitations. First, depending on the time of sampling, there were omissions in the survey area and difficulties in recruiting participants due to the criteria for excluding subjects during the pandemic. For serosurveillance during the COVID-19 pandemic, it is necessary to investigate groups that test routinely and to confirm the history of the diagnosis. To this end, we are conducting additional investigations among military recruits and local communities. Second, the completion date of sample collection (December 12, 2020) might not reflect the third wave of COVID-19 in Korea (late December 2020). Considering the period of antibody production, these findings reflect the prevalence of about 2 weeks before testing. In addition, the formation and duration of antibodies depend on the severity of symptoms [22]. Hence, considering the duration of antibody maintenance, the results might show short-term seroprevalence, rather than a cumulative prevalence. Third, we used only 1 reagent (Roche) in the initial screening test. If more than 1 test had been used for screening, the positivity rate might have been different. Therefore, it is possible that the results were underestimations.
- In conclusion, in Korea, the number of COVID-19 cases was low and there were few hidden undiagnosed infections (asymptomatic patients) in the community, due to the thorough 3T strategy (testing, tracing, treatment). Nevertheless, since a small number of undiagnosed infections may exist in the community, it is necessary to maintain quarantine management and active vaccination.
DISCUSSION
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
-
FUNDING
This study was supported by grant from the Korea National Institute of Health (No. 4861-312).
-
AUTHOR CONTRIBUTIONS
Conceptualization: Kim SS, Lee JW. Data curation: Kim SS, Choi YS, Minn D, Kim DH. Formal analysis: Kim AR, Do HN, Kim SH, Minn D. Funding acquisition: Kwon JW, Kim SS. Methodology: Kim AR, Choi YS, Oh EJ, Oh K, Kim DH. Project administration: Kim SS, Lee JW. Visualization: Kim AR, Do HN, Kim SH, Minn D, Lee JW, Choi YS. Writing-original draft: Kim AR, Lee JW. Writing-review & editing: Kim SS, Kim B, Minn D, Kim SH, Do HN, Choi YS, Kim DH, Oh EJ, Oh K, Kwon D, Kwon JW.
NOTES
ACKNOWLEDGEMENTS
| Collection period | Age/sex |
Roche Elecsys Anti-SARS-CoV-2 assay (ECLIA) |
Architect SARS-CoV-2 IgG assay (CLIA) |
Access SARS-CoV-2 IgG assay |
SG Medical R-FIND COVID-19 ELISA IgG |
SGTi-flex COVID-19 IgM/IgG (lateral flow) |
Plaque reduction neutralizing test |
Result of SARS-CoV-2 PCR test (duration) | Region (domestic or abroad) | Date of sampling (in 2020) | Final result of the antibody against SARS-CoV-21 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | Result | Index | Result | Index | Result | Index | Result | Result | Titer | Result | ||||||
| First | 73/F | 1.15 | + | 0.05 | - | 0.04 | - | 0.38 | - | - | <10.0 | - | NA | Chungbuk | May 21 | - |
| 49/F | 3.13 | + | 0.01 | - | 0.03 | - | 0.62 | - | - | <10.0 | - | NA | Incheon | May 29 | - | |
| 62/M | 1.15 | + | 0.02 | - | 0.03 | - | 0.58 | - | - | <10.0 | - | NA | Incheon | May 29 | - | |
| Second | 41/M | 136 | + | 3.57 | + | 0.36 | - | 0.88 | - | + | 10.7 | + | NA | Seoul | Jun 30 | + |
| 54/F | 1.97 | + | 0.01 | - | 0.02 | - | 0.37 | - | - | <10.0 | - | NA | Seoul | Jul 02 | - | |
| 46/F | 1.74 | + | 0.03 | - | 0.02 | - | 0.50 | - | - | <10.0 | - | NA | Incheon | Jul 08 | - | |
| Third | 27/M | 1.81 | + | 0.04 | - | 0.02 | - | 0.33 | - | - | <10.0 | - | NA | Gangwon | Sep 17 | - |
| 53/M | 70.7 | + | 2.52 | + | 0.44 | - | 0.82 | - | + | 56.5 | + | +(24 wk) | Gyeonggi (abroad) | Sep 26 | + | |
| 35/F | 50.2 | + | 1.43 | + | 0.57 | - | 0.72 | - | - | 95.3 | + | +(24 wk) | Gyeonggi (abroad) | Sep 26 | + | |
| 40/M | 10.8 | + | 0.02 | - | 0.02 | - | 0.61 | - | - | <10.0 | - | NA | Gyeonggi | Oct 08 | - | |
| 61/M | 17.9 | + | 2.23 | + | 0.03 | - | 0.88 | - | - | <10.0 | - | NA | Gangwon | Oct 21 | + | |
| Fourth | 58/F | 1.18 | + | 0.06 | - | 0.06 | - | 0.67 | - | - | <10.0 | - | NA | Gyeongnam | Nov 06 | - |
| 57/F | 35.8 | + | 2.70 | + | 0.93 | - | 0.81 | - | + | 104.4 | + | +(12 wk) | Seoul (domestic) | Dec 08 | + | |
- 1. World Health Organization. COVID-19 – China. 2020 Jan 12 [cited 2021 Dec 1]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON233.
- 2. World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). 2020 Jan 30 [cited 2021 Dec 1]. Available from: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
- 3. World Health Organization. WHO coronavirus (COVID-19) dashboard. [cited 2021 Dec 1]. Available from: https://covid19.who.int/.
- 4. World Health Organization. COVID-19 - Republic of Korea - (ex-China). 2020 Jan 21 [cited 2021 Dec 1]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON238.
- 5. Korea Diseases Control and Prevention Agency. Coronavirus (COVID-19), Republic of Korea. [cited 2021 Dec 1]. Available from: http://ncov.mohw.go.kr/en/tcmBoardView.do?brdId=12&brdGubun=125&dataGubun=&ncvContSeq=4552&contSeq=4552&board_id=&gubun.
- 6. Coronavirus (COVID-19), Republic of Korea. Cases in Korea. [cited 2021 Dec 1]. Available from: http://ncov.mohw.go.kr/en/bdBoardList.do?brdId=16&brdGubun=161&dataGubun=&ncvContSeq=&contSeq=&board_id=&gubun=.
- 7. Government of the Republic of Korea. Tackling COVID-19 health, quarantine and economic measures: Korean experience. 2020 Mar 31 [cited 2021 Dec 1]. Available from: https://ecck.or.kr/wp-content/uploads/2020/03/Tackling-COVID-19-Health-Quarantine-and-Economic-Measures-of-South-Korea.pdf.
- 8. Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect 2021;54:12-16.ArticlePubMed
- 9. Ki M; Task Force for 2019-nCoV. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol Health 2020;42:e2020007.ArticlePubMedPMC
- 10. Park Y, Huh IS, Lee J, Kang CR, Cho SI, Ham HJ, et al. Application of testing-tracing-treatment strategy in response to the COVID19 outbreak in Seoul, Korea. J Korean Med Sci 2020;35:e396.ArticlePubMedPMCPDF
- 11. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69-77.ArticlePubMedPMC
- 12. Duggan J, Otter A, Andrews N, Brooks T. Evaluation of Roche Elecsys antiSARS-CoV-2 S serology assay for the detection of anti-SARS-CoV-2 S antibodies. 2021 Mar 11 [cited 2021 Dec 1]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/989460/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_S_assay_PHE.pdf.
- 13. Bendavid E, Mulaney B, Sood N, Shah S, Bromley-Dulfano R, Lai C, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. Int J Epidemiol 2021;50:410-419.ArticlePubMedPMCPDF
- 14. Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. JAMA 2020;323:2425-2427.ArticlePubMedPMC
- 15. Menachemi N, Yiannoutsos CT, Dixon BE, Duszynski TJ, Fadel WF, Wools-Kaloustian KK, et al. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample - Indiana, April 25-29, 2020. MMWR Morb Mortal Wkly Rep 2020;69:960-964.ArticlePubMedPMC
- 16. Angulo FJ, Finelli L, Swerdlow DL. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open 2021;4:e2033706.ArticlePubMedPMC
- 17. Public Health England. Weekly coronavirus disease 2019 (COVID-19) surveillance report: summary of COVID-19 surveillance systems; week 40. 2020 [cited 2021 Dec 1]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/923668/Weekly_COVID19_Surveillance_Report_week_40.pdf.
- 18. Duan S, Zhou M, Zhang W, Shen J, Qi R, Qin X, et al. Seroprevalence and asymptomatic carrier status of SARS-CoV-2 in Wuhan City and other places of China. PLoS Negl Trop Dis 2021;15:e0008975.ArticlePubMedPMC
- 19. Nawa N, Kuramochi J, Sonoda S, Yamaoka Y, Nukui Y, Miyazaki Y, et al. Seroprevalence of SARS-CoV-2 in Utsunomiya City, Greater Tokyo, after the first pandemic in 2020. J Gen Fam Med 2020;22:160-162.ArticlePubMedPMCPDF
- 20. Nah EH, Cho S, Park H, Hwang I, Cho HI. Nationwide seroprevalence of antibodies to SARS-CoV-2 in asymptomatic population in South Korea: a cross-sectional study. BMJ Open 2021;11:e049837.ArticlePubMed
- 21. U.S. Food and Drug Administration. EUA authorized serology test performance. [cited 2021 Dec 1]. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance.
- 22. Legros V, Denolly S, Vogrig M, Boson B, Siret E, Rigaill J, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol 2021;18:318-327.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Realistic Estimation of COVID-19 Infection by Seroprevalence Surveillance of SARS-CoV-2 Antibodies: An Experience From Korea Metropolitan Area From January to May 2022
In Hwa Jeong, Jong-Hun Kim, Min-Jung Kwon, Jayoung Kim, Hee Jin Huh, Byoungguk Kim, Junewoo Lee, Jeong-hyun Nam, Eun-Suk Kang
Journal of Korean Medical Science.2024;[Epub] CrossRef - Protective effect of vaccination on the risk of cardiovascular disease after SARS-CoV-2 infection
Jihun Song, Seulggie Choi, Seogsong Jeong, Joo young Chang, Sun Jae Park, Yun Hwan Oh, Ji Soo Kim, Yoosun Cho, Kyeonghyang Byeon, Jun Yong Choi, Seju Lee, Sang Min Park
Clinical Research in Cardiology.2023;[Epub] CrossRef - Accelerated Cognitive Function Decline in Community-Dwelling Older Adults during COVID-19 Pandemic: The Korean Frailty and Aging Cohort Study (KFACS)
Jaehoon Jung, Sunyoung Kim, Byungsung Kim, Miji Kim, Jisoo Yang, Dongmin Chung, Changwon Won
International Journal of Environmental Research and Public Health.2022; 19(17): 10666. CrossRef - Seroprevalence of SARS-CoV-2 antibodies during the third wave of COVID-19 in the Seoul metropolitan area of Korea
Kyuhyun Yoon, Jayeun Kim, Kyong Ran Peck, Hyun Soo Kim, Hyukmin Lee, Yoo-Sung Hwang, Soon Young Lee, Sung-il Cho, Hun Jae Lee, Yeong-gyeong Kim, Byoungguk Kim, June-Woo Lee, Ah-Ra Kim, Hyeon Nam Do, Dong-Hyun Kim
Epidemiology and Health.2022; 44: e2022085. CrossRef
- Figure
- Related articles
-
- Development and validation of the Health Literacy Index for the Community (HLIC) for the Korean National Health and Nutrition and Examination Survey
- Association of healthy lifestyle factors with the risk of hypertension, dyslipidemia, and their comorbidity in Korea: results from the Korea National Health and Nutrition Examination Survey 2019-2021
- Mediating effect of lower extremity muscle on the relationship between obesity and osteoarthritis in middle-aged and elderly women in Korea: based on the 2009-2011 Korea National Health and Nutrition Examination Survey
- Folate, vitamin B12, and homocysteine status in the Korean population: data from the 2013-2015 Korea National Health and Nutrition Examination Survey
- Higher energy consumption in the evening is associated with increased odds of obesity and metabolic syndrome: findings from the 2016-2018 Korea National Health and Nutrition Examination Survey (7th KNHANES)

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite


