Testosterone levels and cause-specific mortality in the older French men without metabolic syndrome
Article information
Abstract
OBJECTIVES
Previous studies have reported controversial findings regarding the association of testosterone with mortality in older men. This heterogeneity might be partially explained by comorbidities and the presence of metabolic syndrome, as well as differential associations according to causes of death.
METHODS
We used data from a random subsample of the Three-City study, in which hormone levels were measured in 338 men ≥65 years without metabolic syndrome who were followed-up for 12 years. Vital status was determined for all participants from different sources. We used inverse-probability-weighted Cox regression to estimate the hazard ratios (HRs) of cause-specific mortality and 95% confidence intervals (CIs).
RESULTS
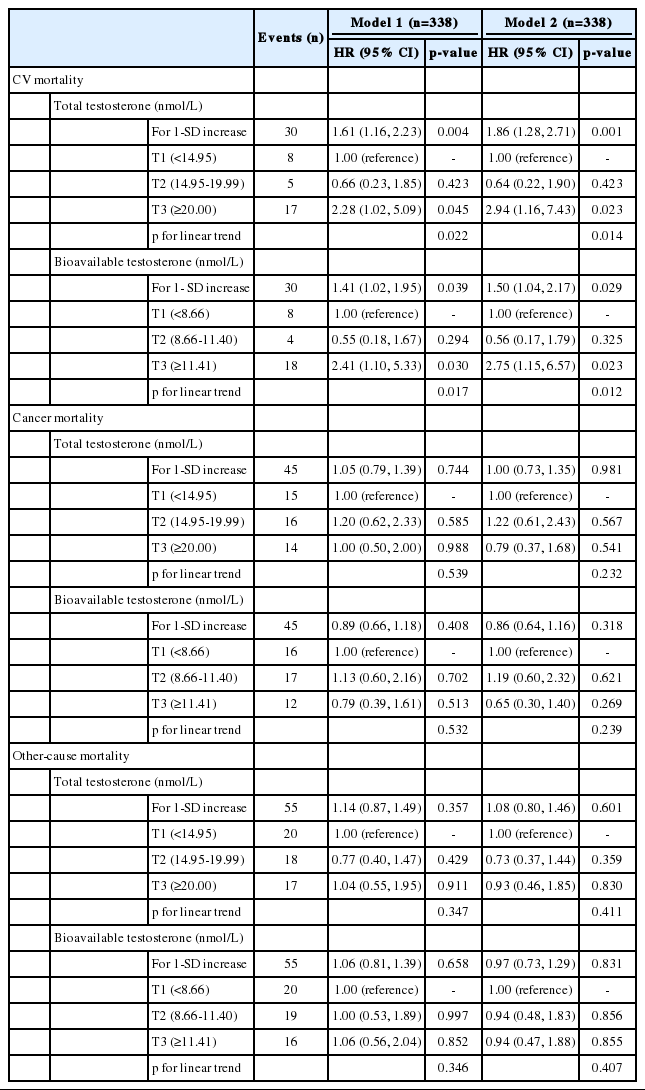
Over the follow-up period, 130 men died (30 from cardiovascular disease, 45 from cancer, 55 from other causes). The association of testosterone with mortality showed significant heterogeneity across causes of death (p=0.027 and p=0.022 for total and bioavailable testosterone, respectively). Higher testosterone levels were associated with increased cardiovascular mortality (HR for 1-standard deviation increase, 1.86; 95% CI, 1.28 to 2.71 and 1.50; 95% CI, 1.04 to 2.17 for total and bioavailable testosterone, respectively). By contrast, there were no significant associations of testosterone with mortality from cancer and other causes.
CONCLUSIONS
Our data suggest that the association of testosterone with mortality in men without metabolic syndrome might be differential according to the cause of death. These findings may partially explain the heterogeneity across studies on the relationship between testosterone levels and mortality.
INTRODUCTION
Findings from studies on the relationship between testosterone levels and mortality in men are highly heterogeneous, making it difficult to draw clear conclusions [1]. Identifying possible sources of heterogeneity may help better understand this relationship and to determine which groups are at higher risk. In a previous analysis, we reported that metabolic syndrome (MetS) could partially explain this heterogeneity, as there was an inverse association between testosterone and all-cause mortality in men with MetS, but not in those without MetS [2]. However, it is possible that testosterone plays a stronger role in specific causes of death, explaining the lack of association between testosterone and all-cause mortality in men without MetS. Indeed, testosterone was positively associated with cardiovascular (CV) events [3,4] but negatively with cancer [5], suggesting differential associations of testosterone with health outcomes. Nevertheless, MetS was not taken into account and no study has examined this differential risk in a metabolically “healthy” population. Specific research that enables the identification of subgroups of men at higher risk would be very important for clinical practice when considering, for example, testosterone therapy or other specific treatments. Therefore, the aim of the present study was to examine the relationship between plasma testosterone and cause-specific mortality in men without MetS.
MATERIALS AND METHODS
The protocol of the Three-City (3C) study, a French prospective cohort study, is available elsewhere [2,6,7]. Briefly, 9,294 non-institutionalized subjects ≥ 65 years of age were selected from the electoral rolls of 3 French cities (Bordeaux, Dijon, and Montpellier) between 1999 and 2001 and interviewed 5 times (every 2 to 3 years) thereafter.
Over the 12-year follow-up, the vital status of each participant was determined based on medical records and reports from the participant’s family and physicians. A committee reviewed these data to determine immediate and underlying causes of death, according to International Classification of Diseases, 10th revision codes. Our analyses are based on the 3 main causes of death: CV, cancer, and other causes, as described elsewhere [7].
Baseline total and bioavailable testosterone were measured in a random subsample representing one-seventh of the men from the original cohort, after stratification by gender, center, and age [2]. Total testosterone was assessed by a radioimmunoassay (RIA) method with an Orion Diagnostica device (Spectria, Espoo, Finland) and a minimum detectable concentration of 0.06 nmol/L; all testosterone assays were detectable. The intra-assay and interassay coefficients of variation were 3.8% and 4.8%, respectively, for a total testosterone concentration of 10.2 nmol/L and 4.8% and 5.5%, respectively, for a total testosterone concentration of 21.3 nmol/L.
MetS was defined according to the Diabetes Federation [2,8]. Body mass index (BMI) was calculated by dividing weight (kg) by squared height (m²). Smoking status and daily alcohol consumption were considered in 3 categories (never, past, current). Education level was categorized into 3 groups (no education or primary school, secondary school, and high-school or university degree). Hypertension was defined according to the World Health Organization criteria (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg and/or blood-pressure-lowering therapy). Hypercholesterolemia was defined as total cholesterol ≥ 6.2 mmol/L and/or cholesterol-lowering therapy, and diabetes as fasting blood glucose ≥ 7 mmol/L and/or antidiabetic drugs. A personal history of CV disease at baseline included coronary heart disease (myocardial infraction, angina pectoris, coronary dilation) and stroke.
The baseline characteristics of the participants are expressed as means± standard deviation (SD), medians (interquartile range), or numbers (percentage) for categorical variables. Their distributions according to causes of death and tertiles of total testosterone were compared using the chi-square test, the Student t-test, or analysis of variance.
We used Cox proportional hazards regression models with age as the time scale to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for cause-specific mortality [9]. Participants were followed-up from baseline to the date of death from a specific cause or censoring (death from another cause or date of last confirmation that they were alive).
Model 1 was adjusted for center; model 2 was further adjusted for confounders (smoking, alcohol drinking, education level, history of CV disease, hypertension, hypercholesterolemia, diabetes, BMI). We created extra categories for covariates with missing values to keep the same number of subjects in the analyses.
In sensitivity analyses, we excluded deaths occurring during the first 2 and 4 years of follow-up.
We used a stabilized inverse-probability-weighting approach to take into account the fact that testosterone was measured in a random subcohort [10]. Weights were calculated for each individual as detailed elsewhere [2].
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics statement
The study protocol was approved by the Ethics Committee of the University Hospital of Kremlin-Bicêtre, and all participants gave written informed consent.
RESULTS
From the random subcohort of 495 men, we excluded 22 men who received treatments that might influence hormone levels or had a history of prostate cancer or prostatitis, 29 men without data on MetS, and 106 men with MetS. The remaining 338 men without MetS constituted the sample for our analysis.
Over 12 years of follow-up, 130 men died (38.5%): 30 from CV disease, 45 from cancer, and 55 from other causes. Compared to survivors, they were older at baseline and more likely to be current smokers and daily alcohol drinkers (for deaths from cancer) than those who were alive at the end of follow-up, independently of their age at inclusion (Table 1). As expected, men with the highest levels of testosterone were less likely to present diabetes (Supplementary Material 1).
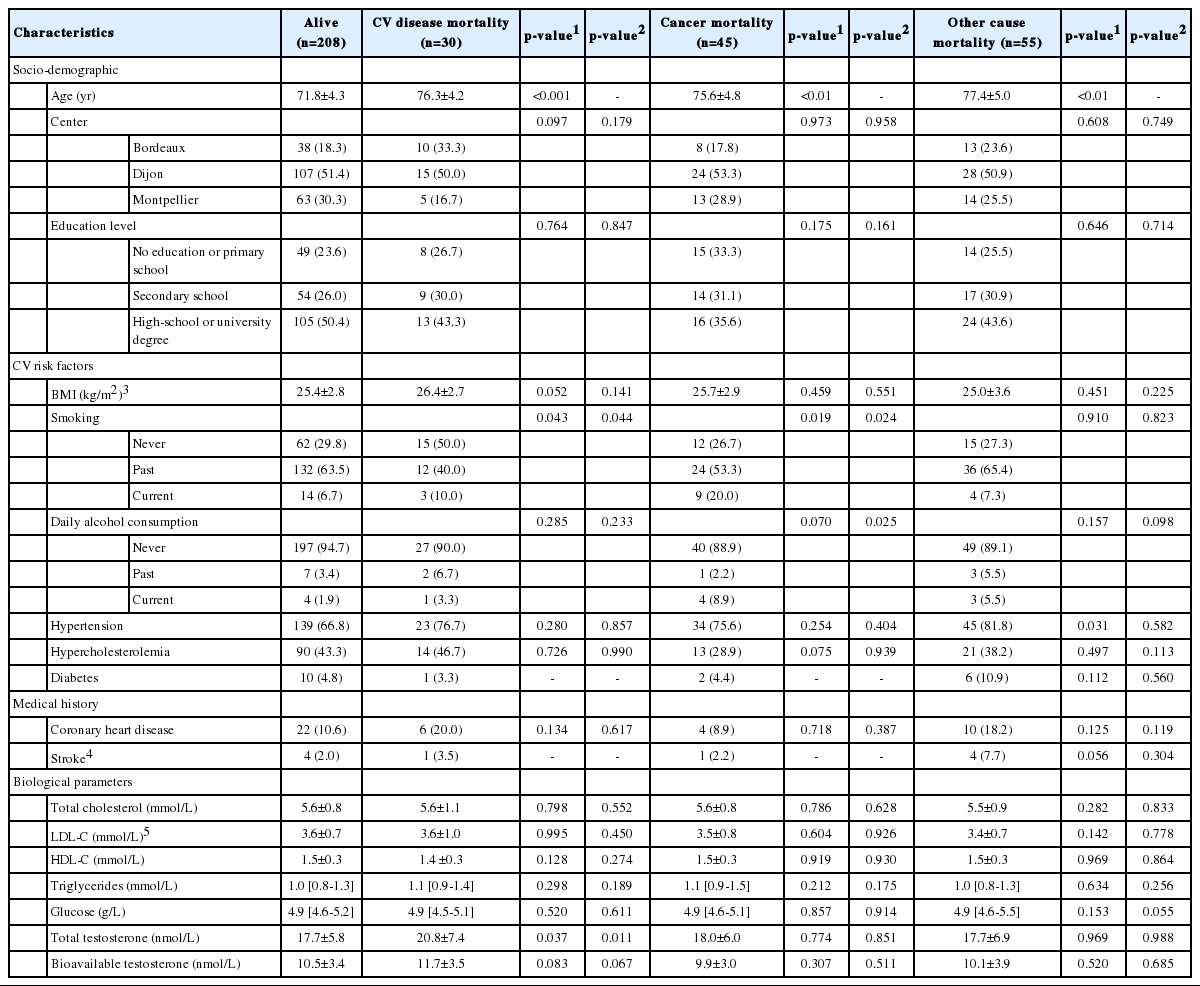
Baseline characteristics of men without MetS according to cause-specific mortality after a 12-year follow-up in the 3C cohort study
Total and bioavailable testosterone were not significantly associated with all-cause mortality in men without MetS (HR for 1-SD increase, 1.08; 95% CI, 0.80 to 1.46 and 0.97; 95% CI, 0.73 to 1.29, respectively) but there was significant heterogeneity across causes of death (p=0.027 and p=0.022, respectively).
The associations of baseline endogenous hormone levels with cause-specific mortality are presented in Table 2. Higher total and bioavailable testosterone levels were positively associated with CV mortality (HR for 1-SD increase, 1.86; 95% CI, 1.28 to 2.71 and 1.50; 95% CI, 1.04 to 2.17, respectively). When testosterone level was categorized into tertiles, we observed a linear trend (p=0.014 and p=0.012, respectively), with a higher incidence of CV death among participants in the top tertile compared to those in the bottom tertile (HR, 2.94; 95% CI, 1.16 to 7.43 and 2.75, 95% CI, 1.15 to 6.57, respectively). There were no significant associations of total and bioavailable testosterone levels with mortality from cancer or other causes.
The results were similar after the exclusion of deaths occurring during the first 2 years and 4 years of follow-up (data not shown).
DISCUSSION
In men without MetS, we found no significant association between testosterone and mortality overall, but we observed a differential association according to the cause of death. Independently of CV risk factors, higher levels of testosterone increased the risk of CV mortality, but were not associated with mortality from cancer and other causes.
This positive association of testosterone with CV mortality differed from some previous studies that showed an inverse association [11-13] or no association [14,15]; however, there are important differences between these studies and ours that could account for these inconsistent findings. First, other studies analyzed younger subjects than those included in the 3C, resulting in lower mortality rates. Second, these studies had shorter follow-up periods, and their findings may have been biased by reverse causation—that is, testosterone levels may have been influenced by the diseases that led to death. Reverse causation is less likely in the 3C study, given the longer follow-up and the findings of our subanalyses with the exclusion of participants who died early during the follow-up. Finally, previous studies did not take MetS status into account, while our analysis was limited to men without MetS who, by definition, have less comorbidities and are healthier compared to men overall. Conversely, our results are consistent with other studies on the risk of death from coronary heart disease [16,17] and CV events [3,4]. This is also in keeping with a meta-analysis of 27 clinical trials (n=2,994 men) showing increased CV risk in men treated with exogenous testosterone compared to placebo [18].
The mechanisms underlying the positive association between testosterone and CV mortality remain unknown. One study hypothesized that high testosterone levels may promote fluid-sodium retention in aging men, thereby contributing to the development of high blood pressure and heart failure [19]. However, in our study, adjustment for hypertension did not have a noteworthy impact. Another study suggested that the use of anabolic steroids is associated with left ventricular hypertrophy [20], thus increasing the risk of CV mortality. Although our data do not concern exogenous testosterone, we cannot exclude a similar mechanism for endogenous testosterone.
With respect to cancer mortality, we found no significant association with testosterone, in agreement with several previous studies [16,21,22]; however, other studies have shown an inverse association between testosterone and risk of cancer mortality [5,23,24]. We could not examine specific cancers due to the low number of cases and we cannot exclude the possibility that some associations might exist for specific cancers. In addition, the people who agreed to participate in the 3C study were healthier than the general population [6], and there were few current smokers at baseline. Since smoking is a very strong risk factor for CV disease and cancer, especially for aero-pharyngeal cancers, this low smoking prevalence may have led to reduced statistical power.
The major limitation of our study is that our analyses were based on a random stratified subcohort of men, rather than on the whole cohort. In order to control for sampling-related biases, we used inverse-probability-weighting [10]. In addition, testosterone measurements were carried out by RIA, rather than the state-of-the-art gas chromatography mass spectrometry method. However, validation studies showed that RIA provided accurate results in terms of relative ranking and was therefore adequate for epidemiological investigations in which population-level inferences are more of interest than subject-specific ones. Finally, the 3C cohort, like most cohort studies, is not representative of the French general population aged 65 years and over. For example, the prevalence of some CV and cancer risk factors, such as smoking status and educational level, was underestimated when compared with the general population [6]. However, we verified well-known associations and therefore we expect that this issue would attenuate, but not bias, the associations. Nevertheless, extrapolating these results to the general population must therefore be done carefully.
In conclusion, among men without MetS, high testosterone levels were found to be associated with increased CV mortality, but not cancer mortality. These findings may partially explain the heterogeneity of studies on the relationship between testosterone and mortality and help clinicians to identify groups at high-risk.
Electronic Supplementary Material
Supplementary materials are available at http://www.e-epih.org/.
Notes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
FUNDING
This work was supported by the Fondation pour la Recherche Médicale for preparation and initiation of the study. The 3C is also supported by the Caisse Nationale d’Assurance Maladie des Travailleurs Salariés, Direction Générale de la Santé, Mutuelle Générale de l’Education Nationale, Institut de la Longévité, Conseils Régionaux of Aquitaine and Bourgogne, Fondation de France, Ministry of Research-INSERM Programme “Cohortes et collections de données biologiques,” Agence Nationale de la Recherche (ANR PNRA 2006 and LongVie 2007), the “Fondation Plan Alzheimer” (FCS 2009-2012), and the Caisse Nationale de Solidarité pour l’Autonomie. The experiments complied with the current laws of the country in which they were performed. Biological assays regarding hemostatic and hormone parameters were supported by a grant from the Agence Nationale de la Recherche (ANR 2007-LVIE-005-01; principal investigator Pierre-Yves Scarabin).
AUTHOR CONTRIBUTIONS
Conceptualization: MC. Data curation: MC. Formal analysis: NL, AE, MC. Funding acquisition: MC. Methodology: MC, AE, NL. Project administration: MC. Visualization: NL, SBT, CH, MLA, CT, AE, AGM, MC. Writing – original draft: NL, AE, MC. Writing – review & editing: NL, SBT, CH, MLA, CT, AE, AGM, MC.
Acknowledgements
None.