Articles
- Page Path
- HOME > Epidemiol Health > Volume 40; 2018 > Article
-
Brief Communication
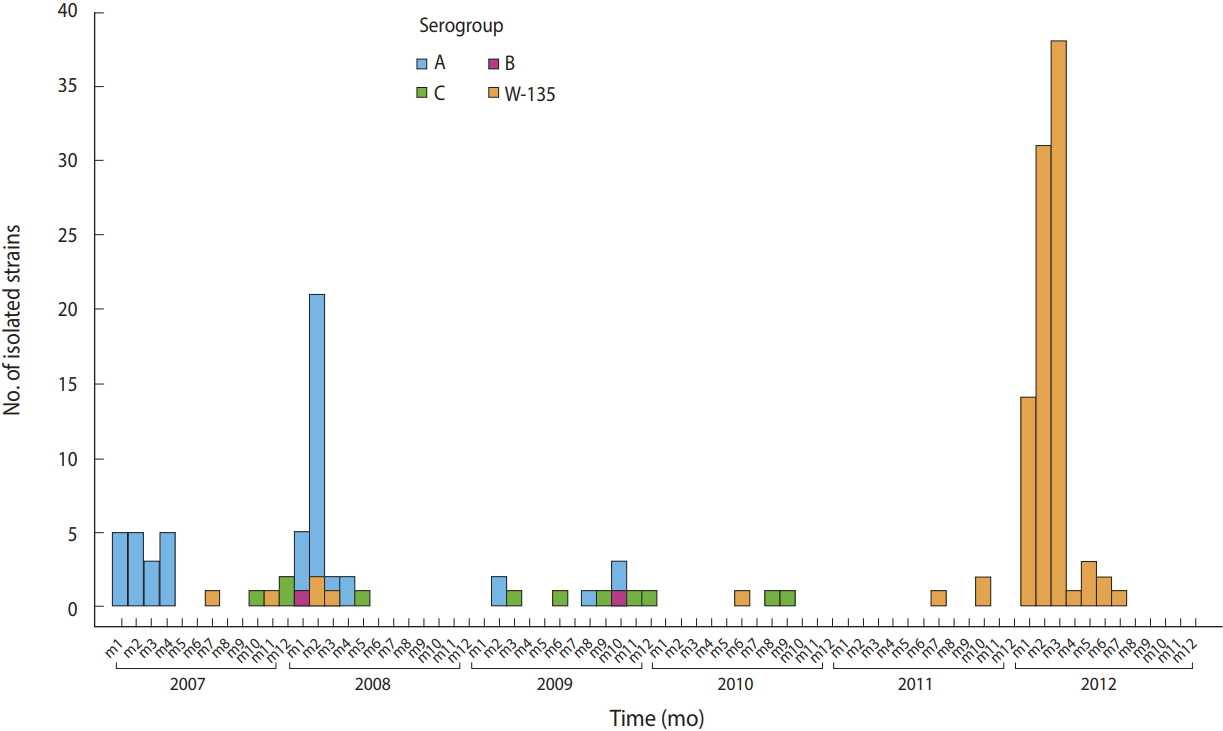
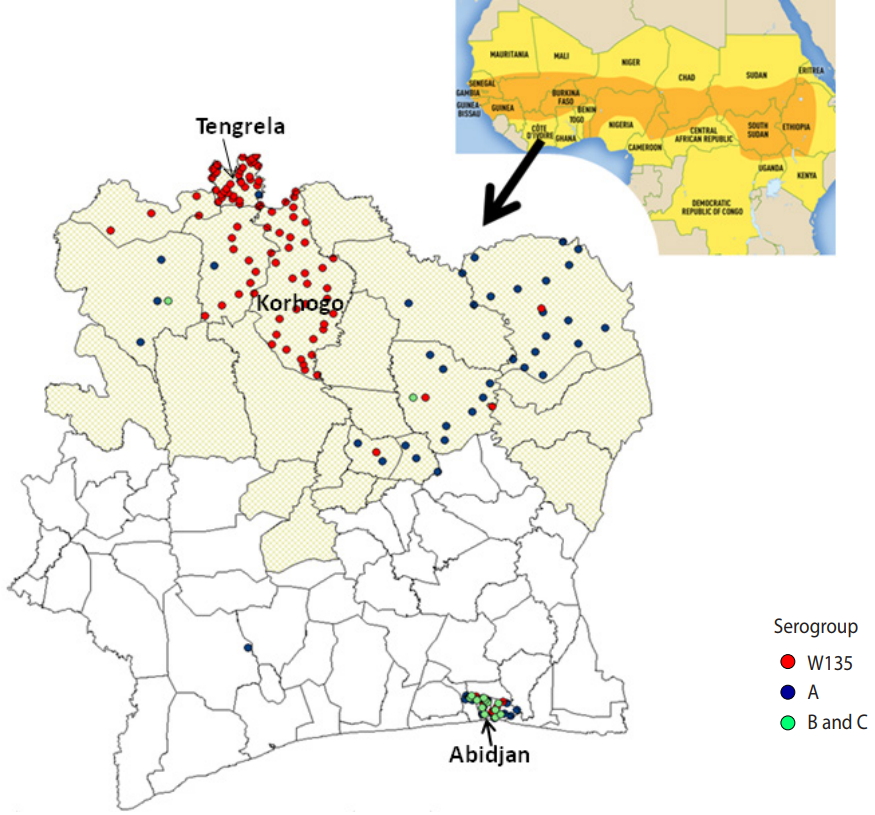
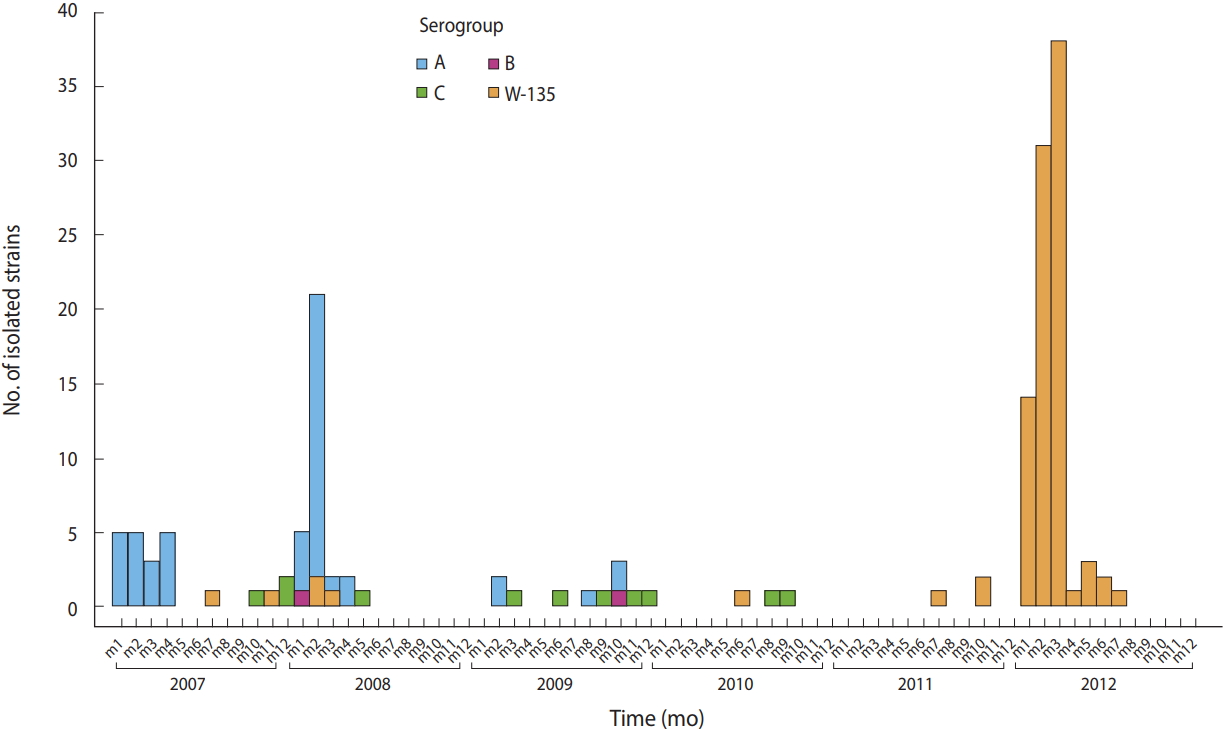
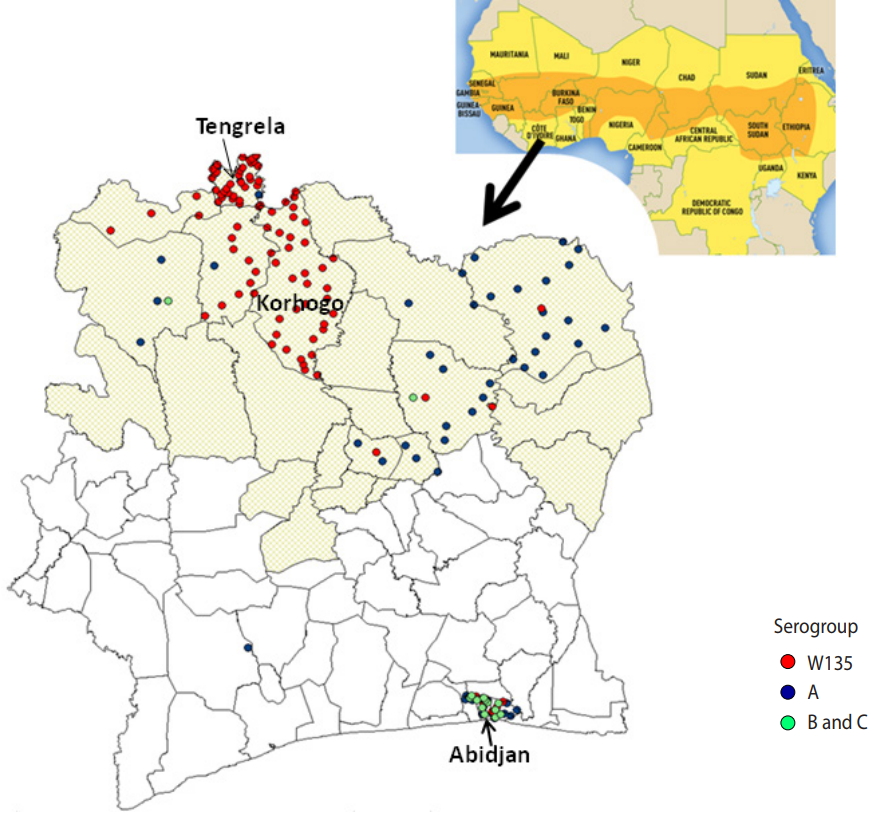
Emergence of Neisseria meningitidis W135 in Cote d’Ivoire: laboratory based-surveillance -
Man-Koumba Soumahoro1
 , Clarisse Kouamé-Elogne2
, Clarisse Kouamé-Elogne2 , Jean-Claude Anné2
, Jean-Claude Anné2 , Soualihou Noufé3
, Soualihou Noufé3 , Kouakou Christophe N’Guessan4
, Kouakou Christophe N’Guessan4 , Adèle Kacou-N’Douba5
, Adèle Kacou-N’Douba5 , Thomas Hanslik6,7
, Thomas Hanslik6,7 , Mireille Dosso2
, Mireille Dosso2
-
Epidemiol Health 2018;40:e2018058.
DOI: https://doi.org/10.4178/epih.e2018058
Published online: November 28, 2018
1Département Epidémiologie Recherche Clinique, Institut Pasteur de Côte d’Ivoire, Abidjan, Cote d’lvoire
2Département Bactériologie Virologie, Institut Pasteur de Côte d’Ivoire, Abidjan, Cote d’lvoire
3Institut National de l’Hygiène Publique, Abidjan, Cote d’lvoire
4Institut National de la Santé Publique, Abidjan, Cote d’lvoire
5Université Félix Houphouët Boigny, Unité de Formation et de Recherche Sciences Médicales, Abidjan, Cote d’lvoire
6Université de Versailles-Saint-Quentin, Montigny-le-Bretonneux, France
7Assistance Publique-Hôpitaux de Paris, Hôpital Ambroise Paré, Service de Médecine Interne, Boulogne Billancourt, France
- Correspondence: Man-Koumba Soumahoro Département Epidémiologie Recherche Clinique, Institut Pasteur de Côte d’Ivoire, 01 BP 490 Abidjan 01, Côte d’Ivoire E-mail: mksoumahoro@pasteur.ci
©2018, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Resurgence of pneumococcal meningitis in Europe and Northern America
D.L.H. Koelman, M.C. Brouwer, D. van de Beek
Clinical Microbiology and Infection.2020; 26(2): 199. CrossRef - Validation of a New Rapid Detection Test for Detection of Neisseria meningitidis A/C/W/X/Y Antigens in Cerebrospinal Fluid
Cyrille H. Haddar, Aude Terrade, Paul Verhoeven, Berthe-Marie Njanpop-Lafourcade, Mireille Dosso, Fati Sidikou, Ali Elhaj Mahamane, Jean-Pierre Lombart, Aziza Razki, Eva Hong, Alain Agnememel, Evelyne Begaud, Yves Germani, Bruno Pozzetto, Muhamed-Kheir Ta
Journal of Clinical Microbiology.2020;[Epub] CrossRef - Epidemiological Characteristics of Meningococcal Meningitis (2016 to 2018) Four Years after the Introduction of Serogroup A Meningococcal Conjugate Vaccine in Benin
Togbemabou Primous Martial Godjedo, Alidehou Jerrold Agbankpe, Moussiliou Noël Paraiso, Tamegnon Victorien Dougnon, Marie Hidjo, Lamine Baba-Moussa, Honore Bankole
Advances in Public Health.2020; 2020: 1. CrossRef

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite