Articles
- Page Path
- HOME > Epidemiol Health > Volume 42; 2020 > Article
-
Systematic Review
Risk factors for stomach cancer: a systematic review and meta-analysis -
Jalal Poorolajal1,2,3
 , Leila Moradi1
, Leila Moradi1 , Younes Mohammadi1,4
, Younes Mohammadi1,4 , Zahra Cheraghi1,3
, Zahra Cheraghi1,3 , Fatemeh Gohari-Ensaf1
, Fatemeh Gohari-Ensaf1
-
Epidemiol Health 2020;42:e2020004.
DOI: https://doi.org/10.4178/epih.e2020004
Published online: February 2, 2020
1Department of Epidemiology, School of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran
2Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
3Modeling of Noncommunicable Diseases Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
4Social Determinants of Health Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
- Correspondence: Leila Moradi Department of Epidemiology, School of Public Health, Hamadan University of Medical Sciences, Hamadan 65157838695, Iran E-mail: leilamoradi073@gmail.com
©2020, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
OBJECTIVES
- This report provides information on 14 behavioral and nutritional factors that can be addressed in stomach cancer prevention programs.
-
METHODS
- PubMed, Web of Science, and Scopus were searched through December 2018. Reference lists were also screened. Observational studies addressing the associations between stomach cancer and behavioral factors were analyzed. Between-study heterogeneity was investigated using the χ2, τ2, and I2 statistics. The likelihood of publication bias was explored using the Begg and Egger tests and trim-and-fill analysis. Effect sizes were expressed as odds ratios (ORs) with 95% confidence intervals (CIs) using a random-effects model.
-
RESULTS
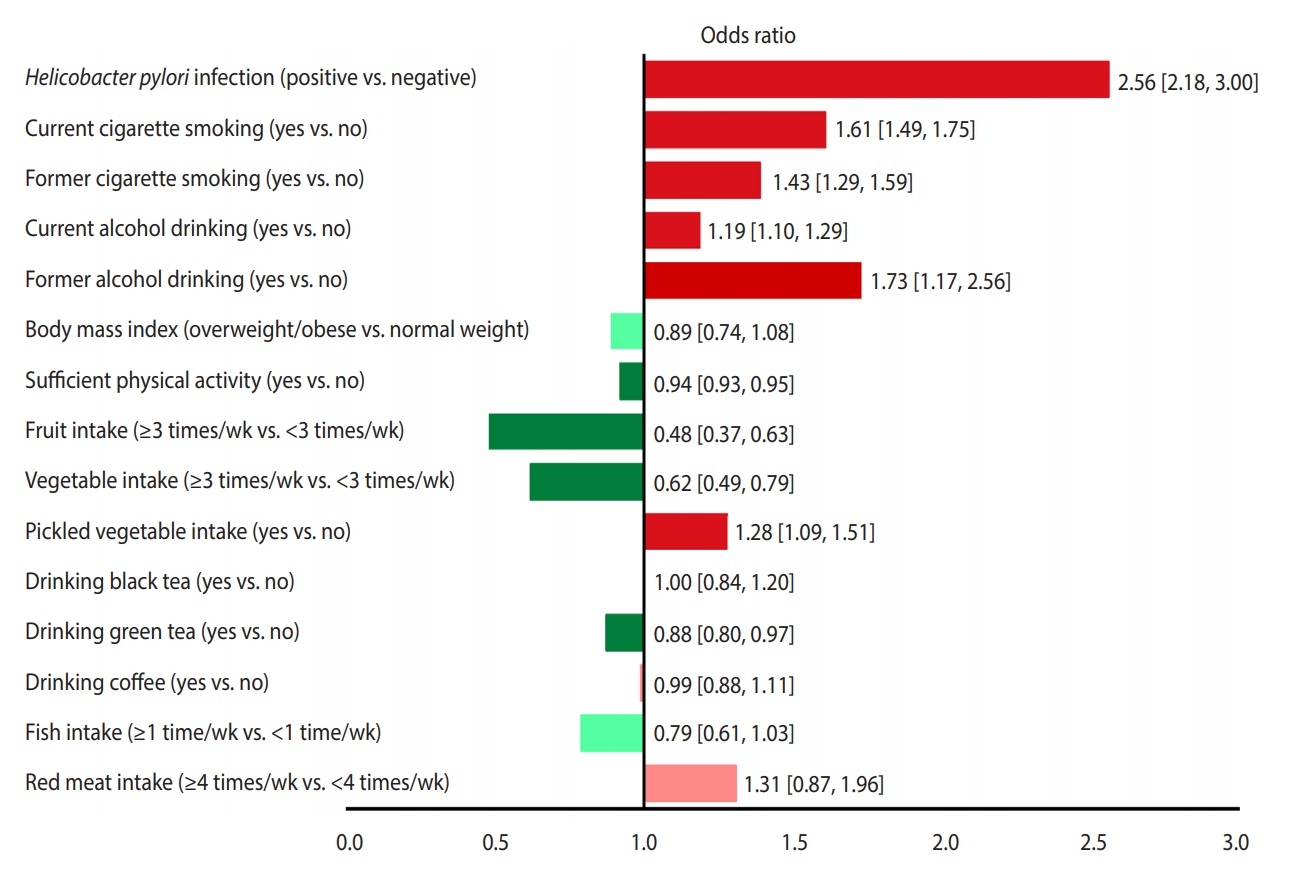
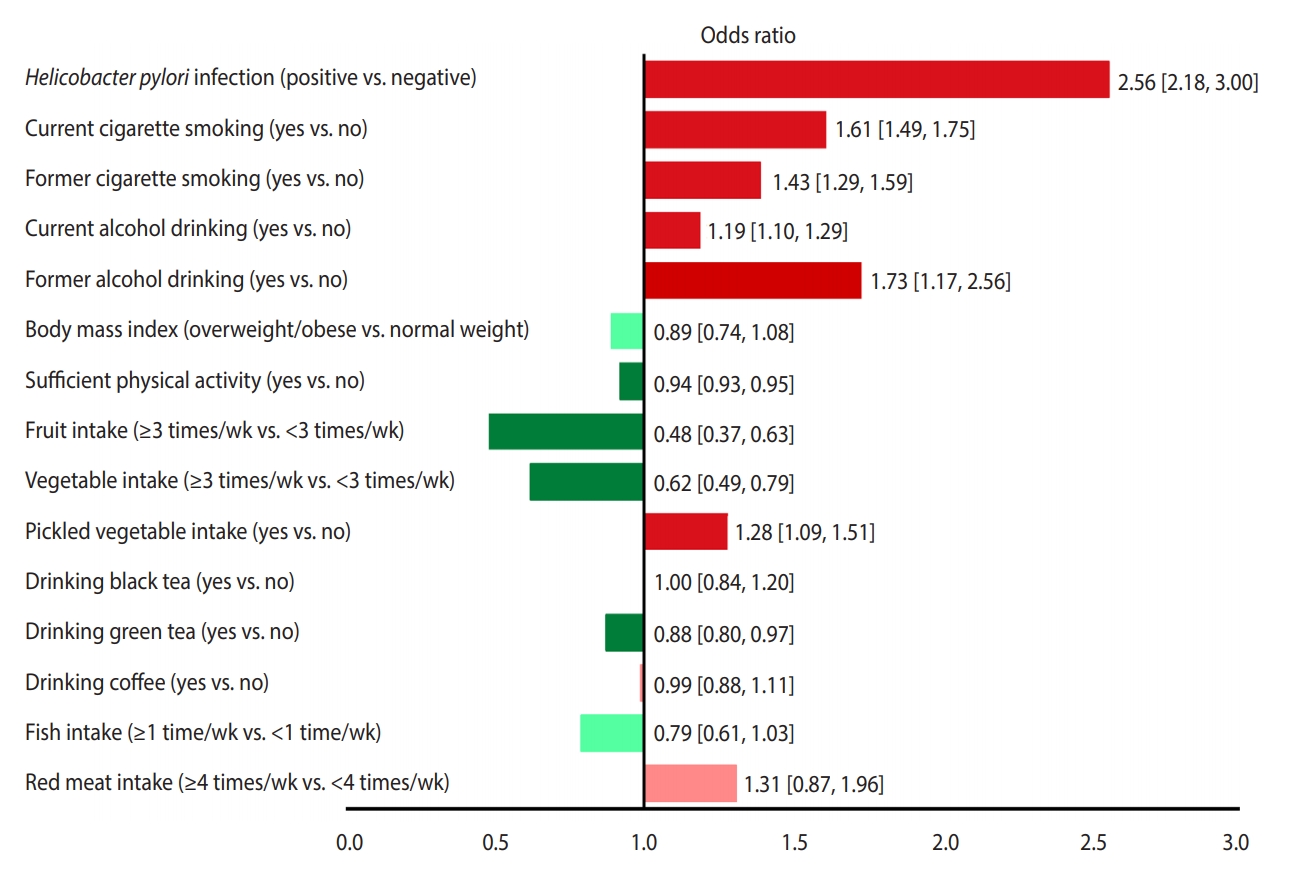
- Of 52,916 identified studies, 232 (including 33,831,063 participants) were eligible. The OR (95% CI) of factors associated with stomach cancer were as follows: Helicobacter pylori infection, 2.56 (95% CI, 2.18 to 3.00); current smoking, 1.61 (95% CI, 1.49 to 1.75); former smoking 1.43 (95% CI, 1.29 to 1.59); current drinking, 1.19 (95% CI, 1.10 to 1.29); former drinking, 1.73 (95% CI, 1.17 to 2.56); overweight/obesity, 0.89 (95% CI, 0.74 to 1.08); sufficient physical activity, 0.83 (95% CI, 0.68 to 1.02); consumption of fruits ≥3 times/wk, 0.48 (95% CI, 0.37 to 0.63); consumption of vegetables ≥3 times/wk, 0.62 (95% CI, 0.49 to 0.79); eating pickled vegetables, 1.28 (95% CI, 1.09 to 1.51); drinking black tea, 1.00 (95% CI, 0.84 to 1.20); drinking green tea, 0.88 (95% CI, 0.80 to 0.97); drinking coffee, 0.99 (95% CI, 0.88 to 1.11); eating fish ≥1 time/wk 0.79 (95% CI, 0.61 to 1.03); eating red meat ≥4 times/wk 1.31 (95% CI, 0.87 to 1.96), and high salt intake 3.78 (95% CI, 1.74 to 5.44) and 1.34 (95% CI, 0.88 to 2.03), based on two different studies.
-
CONCLUSIONS
- This meta-analysis provided a clear picture of the behavioral and nutritional factors associated with the development of stomach cancer. These results may be utilized for ranking and prioritizing preventable risk factors to implement effective prevention programs.
- Stomach cancer, also known as gastric cancer, is the fifth most frequent type of cancer and the third-leading cause of cancer-related death worldwide, responsible for over 1,000,000 new cases and an estimated 783,000 deaths in 2018 [1].
- Many factors may play a role in the development of stomach cancer. Advanced age [2], male sex [1], ethnicity [3], and genetic factors [4] may contribute to the development of stomach cancer, but they are neither modifiable nor preventable. However, nutritional factors [5] and behavioral factors such as cigarette smoking [6] and drinking alcohol [6,7], as well as Helicobacter pylori infection [8], also contribute to the development of stomach cancer. These factors are largely modifiable and preventable, and therefore can be considered when designing effective prevention programs.
- Efforts to improve screening programs and the early detection and treatment of stomach cancer are important, but taking action to address preventable factors that play a role in the development of stomach cancer is a priority. Ranking and prioritizing the factors that contribute to stomach cancer and implementing prevention programs can prevent thousands of cases of stomach cancer each year. Effective intervention strategies and prevention programs require a comprehensive understanding and a clear picture of the factors that promote stomach cancer. No comprehensive systematic review has yet been conducted to address all the potential behavioral and nutritional factors that play a pivotal role in the development of stomach cancer. This systematic review was conducted to address the associations between stomach cancer and 14 potentially modifiable behavioral and nutritional factors that may be addressed in prevention programs aimed at reducing the incidence of stomach cancer.
INTRODUCTION
- Eligibility criteria
- The outcome of interest was pathologically confirmed stomach cancer, of any type (adenocarcinoma, lymphoma, sarcoma, or carcinoid) and location (cardia or non-cardia), among the general population, regardless of age, sex, race, ethnicity, and geographical region. The exposures of interest are listed below:
- H. pylori infection, regardless of cytotoxin-associated gene A (CagA) pathogenicity (positive vs. negative); Cigarette smoking (current/former smokers vs. non-smokers); Drinking alcohol (current/former drinkers vs. non-drinkers); Body mass index (BMI; overweight/obese vs. normal weight); Physical activity (sufficient vs. insufficient); Fruit consumption (≥7 times/wk vs. <7 times/wk and ≥3 times/wk vs. <3 times/wk); Vegetable consumption (≥7 times/wk vs. <7 times/wk and ≥3 times/wk vs. <3 times/wk); Consumption of pickled vegetables (yes vs. no); Drinking black tea (yes vs. no); Drinking green tea (yes vs. no); Drinking coffee (yes vs. no); Fish consumption (≥1 serving/wk vs. <1 serving/wk); Red meat consumption (≥4 times/wk vs. <4 times/wk); Salt intake (>5 g/d vs. ≤5 g/d); A BMI of 18.5-24.9 kg/m2 was classified as normal weight, 25.0-29.9 kg/m2 as overweight, and ≥30.0 kg/m2 as obese.
- At least 60 minutes of moderate- to vigorous-intensity physical activity per day (or 300 min/wk) was considered sufficient for adults [9].
- Observational (cohort and case-control) studies addressing the association between stomach cancer and any of the above factors were included in the meta-analysis, irrespective of language, publication date, and the nationality, race, sex, and age of participants.
- Information sources and search
- PubMed, Web of Science, and Scopus were searched through December 2018. The reference lists of the included studies were also explored. The following terms were searched: (stomach cancer OR gastric cancer OR stomach neoplasms OR gastric neoplasms OR gastric malignancy OR stomach malignancy OR stomach tumor OR gastric tumor) AND (Helicobacter pylori OR H. pylori OR smoking OR cigarette OR tobacco products OR tobacco OR alcohol OR ethanol OR body mass index OR BMI OR overweight OR obesity OR obese OR physical activity OR exercise OR fruit OR vegetable OR pickled OR meat OR coffee OR tea OR fish OR salt OR sodium chloride).
- Study selection
- The search results of all databases were combined using EndNote, and duplicates were deleted. Then, 2 authors (LM and FG) independently screened the titles and abstracts and excluded ineligible studies. The full texts of potentially relevant studies were retrieved for further evaluation.
- Data extraction
- The data from the relevant studies were extracted by 2 authors (LM and JP) using an electronic data collection form prepared in Stata (StataCorp., College Station, TX, USA).
- Methodological quality
- The Newcastle-Ottawa Scale (NOS) [10] was used to assess the methodological quality of the included studies. Based on this scale, a maximum of 9 stars was assigned to each study. Studies that received 7 or more stars were labeled high-quality, and otherwise studies were classified as low-quality.
- Heterogeneity and publication bias
- The heterogeneity across studies was examined using the chi-square (χ2) test [11] and tau-square (τ2) test and was quantified by the I2 statistic [12]. According to the I2 value, heterogeneity was classified as low (<50%), moderate (50-74%), or high (≥75%).
- The possibility of publication bias was explored by the Egger [13] and Begg [14] tests and the trim-and-fill method [15].
- Summary measures
- The effect measure of choice was the odds ratio (OR), rate ratio, or hazard ratio with 95% confidence intervals (CIs). However, we analyzed these effect measures separately.
- The results were reported based on a random-effects model [16]. The data were analyzed at a significance level of 0.05 using Stata version 14.2 (StataCorp., College Station, TX, USA) and Review Manager version 5.3 (https://review-manager.software.informer.com/5.3/).
- Sensitivity analysis
- If the between-study heterogeneity was moderate to high (I2 ≥50%), the source of heterogeneity was investigated using a sequential algorithm [17].
- Ethics statement
- This study was a systematic review in which no human subject or animal was employed.
MATERIALS AND METHODS
- Description of studies
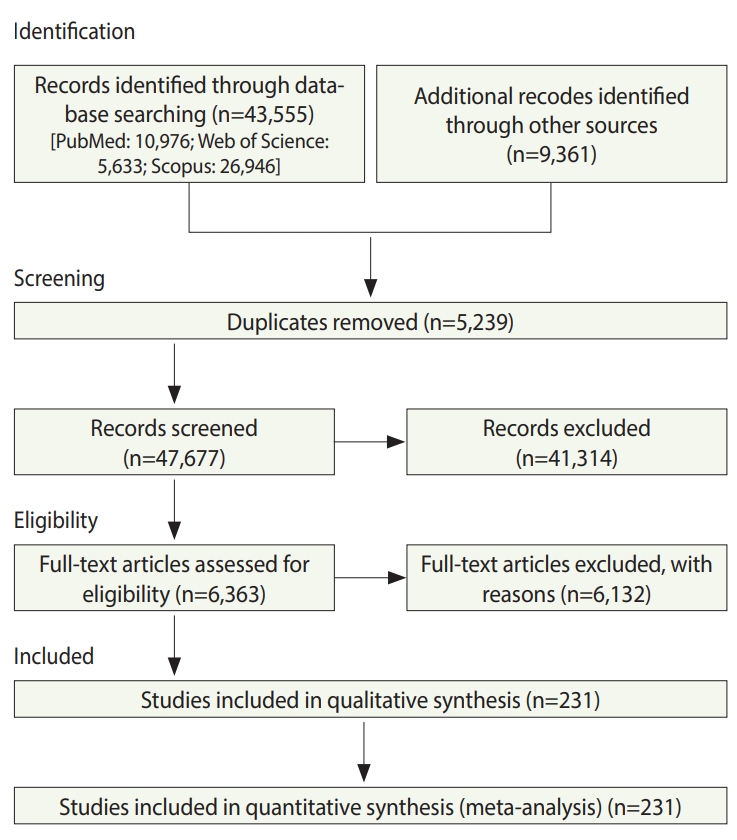
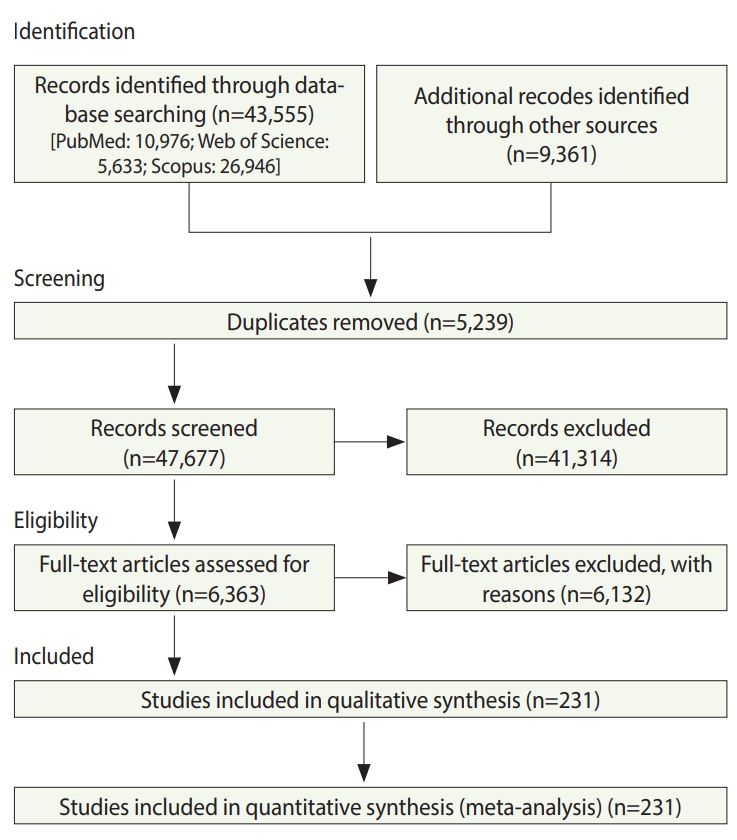
- In total, 52,916 studies were identified, including 43,555 studies obtained by searching the electronic databases through December 2018 and 9,359 articles identified by searching the reference lists of the included studies. After excluding duplicates and ineligible studies, 232 studies with 33,831,063 participants (Supplementary Material 1) were included in the meta-analysis (Figure 1).
- Synthesis of results
- Based on 68 studies (Supplementary Material 2), the overall OR for positive versus negative H. pylori infection status was 2.56 (95% CI, 2.18 to 3.00). The overall effect measure showed that H. pylori infection significantly increased the risk of stomach cancer by more than 2.5-fold (p=0.001). Between-study heterogeneity was high (I2=86%). The overall effect became weaker (OR, 2.13; 95% CI, 1.89 to 2.41; I2=47%) after performing a sensitivity analysis (Table 1).
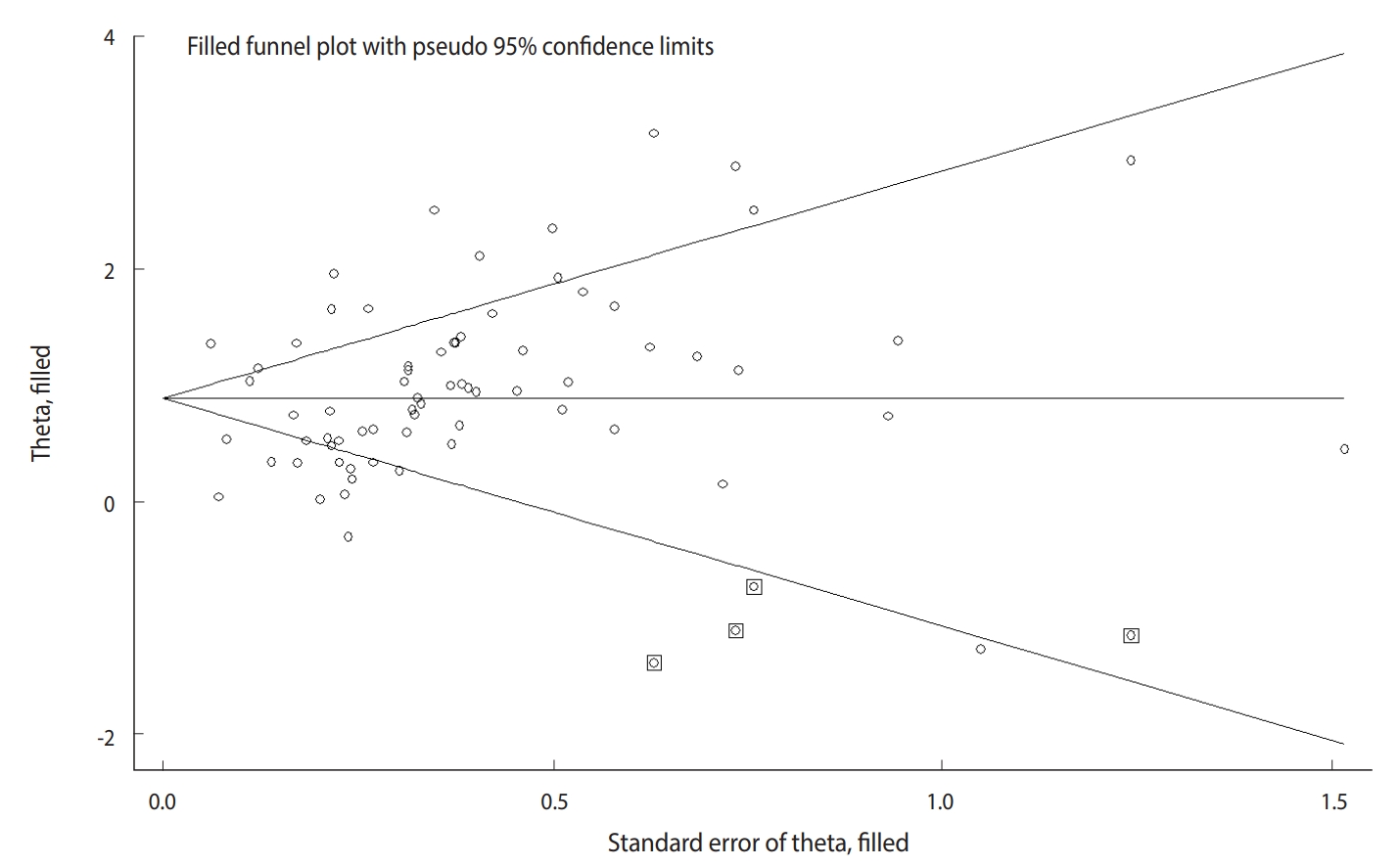
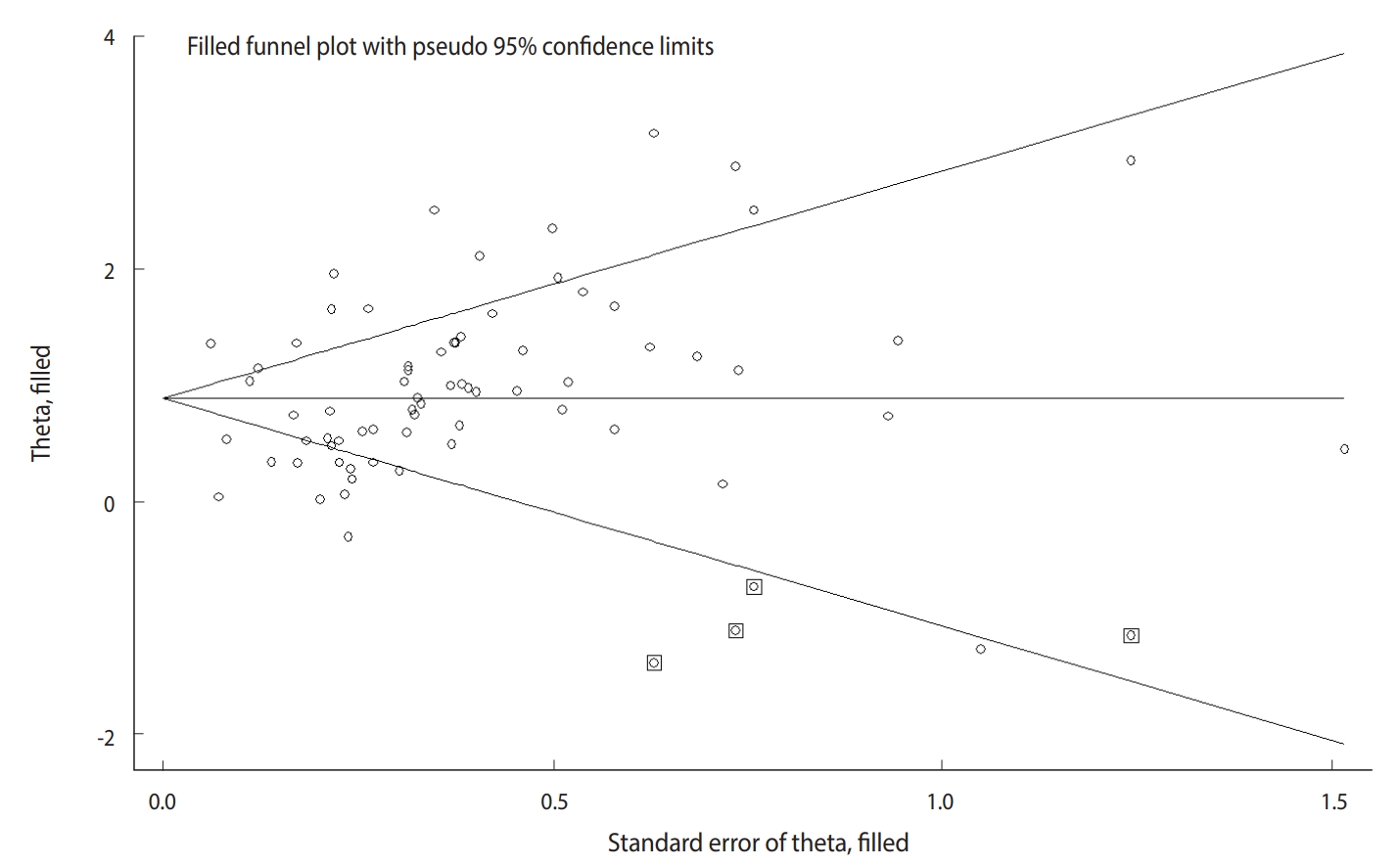
- The Begg test (p=0.004), but not the Egger test (p=0.122), revealed evidence of publication bias. Trim-and-fill analysis estimated 4 missing studies (Figure 2). The overall effect measure based on this analysis was an OR of 2.42 (95% CI, 2.06 to 2.83), which was slightly weaker than the originally reported overall effect measure.
- Based on 77 studies (Supplementary Material 3), the overall OR for current smokers versus never smokers was 1.61 (95% CI, 1.49 to 1.75). The overall effect measure showed that current smoking significantly increased the risk of stomach cancer by 61% (p=0.001). Between-study heterogeneity was high (I2=78%). The overall effect became slightly stronger (OR, 1.66; 95% CI, 1.54 to 1.79; I2=49%) after performing a sensitivity analysis (Table 1).
- In addition, based on 66 studies (Supplemental Material 4), the overall OR for former smokers versus never smokers was 1.43 (95% CI, 1.29 to 1.59). The overall effect measure showed that former smoking significantly increased the risk of stomach cancer by 43% (p=0.001). Between-study heterogeneity was moderate (I2=65%). The overall effect became slightly weaker (OR, 1.35; 95% CI, 1.24 to 1.47; I2=44%) after performing a sensitivity analysis (Table 1).
- The Begg test revealed no evidence of publication bias (p=0.722), but the Egger test did show evidence of publication bias (p=0.001). Trim-and-fill analysis estimated 19 missing studies, but the overall effect measure did not change significantly.
- Based on 84 studies (Supplementary Material 5), the overall OR for current drinkers versus never drinkers was 1.19 (95% CI, 1.10, 1.29). The overall effect measure showed that current drinking significantly increased the risk of stomach cancer by 19% (p= 0.001). Between-study heterogeneity was high (I2=83%). The overall effect became slightly weaker (OR, 1.05; 95% CI, 0.99 to 1.11; I2=50%) after performing a sensitivity analysis (Table 1).
- In addition, based on 16 studies (Supplementary Material 6), the overall OR for former drinking versus never drinking was 1.73 (95% CI, 1.17 to 2.56). The overall effect measure showed that former drinking significantly increased the risk of stomach cancer by 73% (p=0.004). Between-study heterogeneity was high (I2= 84%). The overall effect became slightly weaker (OR, 2.01; 95% CI, 1.48 to 2.72; I2=48%) after performing a sensitivity analysis (Table 1). There was no evidence of publication bias.
- Based on 25 studies (Supplementary Material 7), the overall OR for overweight/obesity versus normal weight was 0.89 (95% CI, 0.74 to 1.08). The overall effect measure showed that overweight/obesity had no significant effect on stomach cancer (p=0.240). Between-study heterogeneity was high (I2=86%). The overall effect changed slightly (OR, 1.14; 95% CI, 1.03 to 1.26; I2=41%) after performing a sensitivity analysis (Table 1). There was no evidence of publication bias.
- Based on 11 studies (Supplementary Material 8), the overall OR for sufficient versus insufficient physical activity was 0.83 (95% CI, 0.68 to 1.02). The overall effect measure showed that physical activity had no significant effect on stomach cancer (p=0.080), which seems negligible. Between-study heterogeneity was low (I2=45%). There was no evidence of publication bias.
- Based on 13 studies (Supplementary Material 9), the overall OR for fruit consumption ≥3 times/wk versus fruit consumption <3 times/wk was 0.48 (95% CI, 0.37 to 0.63). The overall effect measure showed that fruit consumption significantly reduced the risk of stomach cancer by 48% (p=0.001). Between-study heterogeneity was high (I2=86%). The overall effect became slightly weaker (OR, 0.64; 95% CI, 0.55 to 0.75; I2=42%) after performing a sensitivity analysis (Table 1). Both the Begg test (p=0.010) and the Egger test (p=0.001) revealed evidence of publication bias, but trim-and-fill analysis did not change the results.
- Based on 18 studies (Supplementary Material 10), the OR for vegetable consumption ≥3 times/wk versus vegetable consumption <3 times/wk was 0.62 (95% CI, 0.49 to 0.79). The overall effect measure showed that vegetable consumption significantly reduced the risk of stomach cancer by 62% (p=0.001). Between-study heterogeneity was high (I2=74%). The overall effect became slightly weaker (OR, 0.70; 95% CI, 0.58 to 0.84; I2=40%) after performing a sensitivity analysis (Table 1). There was no evidence of publication bias.
- Based on 19 studies (Supplementary Material 11), the overall OR for consuming versus not consuming pickled vegetables was 1.28 (95% CI, 1.09 to 1.51). The overall effect measure showed that consuming pickled vegetables significantly increased the risk of stomach cancer by 28% (p=0.001). Between-study heterogeneity was low (I2=39%). There was no evidence of publication bias.
- Based on 15 studies (Supplementary Material 12), the overall OR for drinking versus not drinking black tea was 1.00 (95% CI, 0.84 to 1.20). The overall effect measure showed that drinking black tea had no significant effect on stomach cancer (p=0.970). Between-study heterogeneity was moderate (I2=62%). The overall effect became slightly stronger (OR, 0.94; 95% CI, 0.83 to 1.07; I2=34%) after performing a sensitivity analysis (Table 1). No evidence of publication bias was revealed.
- Based on 16 studies (Supplementary Material 13), the overall OR for drinking versus not drinking green tea was 0.88 (95% CI, 0.80 to 0.97). The overall effect measure showed that drinking green tea had no significant effect on stomach cancer (p=0.010). Between-study heterogeneity was low (I2=22%). No evidence of publication bias was seen.
- Based on 14 studies (Supplementary Material 14), the overall OR for drinking coffee versus not drinking coffee was 0.99 (95% CI, 0.88 to 1.11). The overall effect measure showed that coffee drinking had no significant effect on stomach cancer (p=0.820). Between-study heterogeneity was moderate (I2=29%). There was no evidence of publication bias.
- Based on 11 studies (Supplementary Material 15), the OR for eating fish ≥1 time/wk versus <1 time/wk was 0.79 (95% CI, 0.61 to 1.03). The overall effect showed that fish consumption had no significant effect on stomach cancer (p=0.080). Between-study heterogeneity was high (I2=76%). The overall effect became stronger (OR, 0.68; 95% CI, 0.55 to 0.83; I2=45%) after performing a sensitivity analysis (Table 1). No evidence of publication bias was seen.
- Based on 11 studies (Supplementary Material 16), the overall OR for eating red meat ≥4 times/wk versus <4 times/wk was 1.31 (95% CI, 0.87 to 1.96). The overall effect measure showed that consumption of red meat had no significant effect on stomach cancer (p=0.080). Between-study heterogeneity was high (I2=83%). The overall effect changed slightly (OR, 0.91; 95% CI, 0.77 to 1.09; I2= 11%) after performing a sensitivity analysis (Table 1). There was no evidence of publication bias.
- Only 2 studies addressed the association between high salt intake and stomach cancer. The results of the two studies are reported separately rather than a pooled OR because of the number of studies was limited. According to these studies, the OR for salt intake of >5 g/d versus ≤5 g/d was 3.78 (95% CI, 1.74 to 5.44) [18] and 1.34 (95% CI, 0.88 to 2.03) [19], respectively. Both studies reported that a high intake of salt significantly increased the risk of stomach cancer.
- Figure 3 presents a unified overview of the associations between stomach cancer and all nutritional and behavioral factors. As shown in this figure, H. pylori infection, current and former cigarette smoking, current and former alcohol drinking, and pickled vegetable consumption were found to significantly increase the risk of stomach cancer. In contrast, sufficient physical activity, fruit consumption, and vegetable consumption significantly reduced the risk of stomach cancer. Meanwhile, BMI, drinking black tea, green tea, and coffee, and eating fish and red meat had no statistically significant effects on the risk of stomach cancer.
RESULTS
H. pylori infection
Cigarette smoking
Drinking alcohol
Body mass index
Sufficient physical activity
Fruits
Vegetables
Pickled vegetable
Black tea
Green tea
Coffee
Fish
Red meat
Salt
- According to our findings, H. pylori infection and smoking were the first and second most powerful risk factors for stomach cancer, respectively, whereas fruit and vegetable consumption were the first and second most powerful protective factors against stomach cancer, respectively.
- The magnitudes of the measures of association reported in this systematic review may be used for ranking and prioritizing the relative importance of risk and protective factors. However, it should be kept in mind that these factors vary in terms of their physiological modus operandi and their units of exposure. Therefore, direct comparisons are often unwarranted [20]. In other words, the mere fact that the ORs of some risk factors for stomach cancer are higher than the ORs of other risk factors is not a sufficient basis for ranking and prioritizing risk factors. Instead, the prevalence of risk factors in the community is an essential criterion that must be taken into account when ranking and prioritizing risk factors. When the association between a particular risk factor and the outcome of interest is strong (a high OR), but the prevalence of that risk factor is low in the community, the overall impact of the risk factor on the disease burden in the community is low. In contrast, when a particular risk factor is common in the community, the overall impact of the factor on the outcome of interest may be tremendous even if the association between the risk factor and the outcome is not as strong (a low OR). Therefore, ranking and prioritizing the behavioral and nutritional factors affecting stomach cancer risk depends on both the strength of the associations (the magnitude of ORs) and the prevalence of the factors in the community.
- Our results indicated that H. pylori infection was strongly associated with the development of stomach cancer. Based on the available evidence, H. pylori infection induces stomach cancer through direct and indirect pathways. The direct action of H. pylori on gastric epithelial cells is thought to be mediated by the induction of protein modulation and genetic mutations. Its indirect action on gastric epithelial cells is thought to be through inflammation. Both pathways work together to promote gastric carcinogenesis [21]. In addition, CagA apparently interacts with some host proteins that regulate cell growth, cell motility, and cell polarity. These interactions with CagA induce morphological transformations that may predispose cells to epigenetic changes involved in gastric carcinogenesis [22].
- Our results revealed a positive relationship between cigarette smoking and the development of stomach cancer. Cigarette smoke contains over 7,000 toxic chemicals, including human carcinogens [23]. These toxins and carcinogens can cause direct DNA damage. Since DNA controls cells’ normal growth and function, DNA damage can alter cells’ growth patterns, and abnormal gastric epithelial cells with DNA damage can turn into cancer [24,25].
- This systematic review showed that drinking alcohol increased the risk of developing stomach cancer. Acetaldehyde, the first and most toxic metabolite of ethanol, is a human carcinogen that can induce DNA lesions by inhibiting DNA methylation and by interacting with retinoid metabolism [26]. DNA lesions may lead to cell mutations, which convert a normal cell into cancer [27]. In addition, alcohol can act as an irritant and cause mucosal damage. The damaged cells may try to repair themselves, which could lead to DNA changes that can be a step toward cancer [28].
- According to our results, the risk of stomach cancer of former drinkers was higher than that of current drinkers. One possible explanation for this finding is that former drinkers might be heavy drinkers who had drunk alcohol for many years, but were forced to quit drinking alcohol because of severe liver and gastric complications.
- Pickled vegetables may increase the risk of stomach cancer because they contain large amounts of salt and because key nutrients are lost in vegetables under acidic and oxygenic conditions [29,30]. Furthermore, pickled vegetables are considered to be a possible source of nitrosamines, which may contribute to gastric carcinogenesis. The contamination of pickled vegetables with fungi has also been postulated to contribute to the incidence of stomach cancer [31].
- Based on our findings, fruit and vegetable consumption was associated with a substantial reduction in stomach cancer risk. It has been postulated that the anti-carcinogenic effects of fruits and vegetables may be attributed to the antioxidant effect of their vitamin content, especially vitamin C and beta-carotene. Antioxidants neutralize reactive oxygen free radicals, which cause DNA damage [32,33]. Damaged DNA may lead to genetic modifications and carcinogenesis [24,25].
- Our results showed a protective, but non-significant accusation between stomach cancer and overweight and obesity. However, the between-study heterogeneity was high (I2=86%). When we performed a sensitivity analysis, the overall effect changed from protective to a significant risk elevation (OR, 1.14; 95% CI, 1.03 to 1.26). Chen et al. [34]. conducted a meta-analysis including studies published before 2013 that were indexed in MEDLINE and EMBASE to address the association between gastric cancer and BMI. They reported that the relative risk of gastric cancer was 1.01 (95% CI, 0.96 to 1.07) for overweight and 1.06 (95% CI, 0.99 to 1.12) for obesity, and neither of those associations was statistically significant. Based on the current evidence, BMI does not seem to have a significant effect on the incidence of stomach cancer.
- This systematic review has a few limitations and potential biases. There were some studies, mostly old, that seemed potentially eligible to be included in this meta-analysis, but neither their full texts nor their corresponding authors were accessible. This issue might have introduced selection bias in our results. Furthermore, several epidemiological studies that investigated the associations between stomach cancer and some nutritional and behavioral risk factors were excluded from the meta-analysis because they were not consistent with the inclusion criteria of this review. This issue may also raise the possibility of selection bias.
DISCUSSION
- This meta-analysis provided a clear picture of several behavioral and nutritional factors that play pivotal roles in the development of stomach cancer. These results are helpful and may be utilized for ranking and prioritizing preventable risk factors to implement effective interventions and community-based prevention programs. We reemphasize that both the strength of associations and the prevalence of factors in the community should be taken into account when ranking and prioritizing stomach cancer–associated factors.
CONCLUSION
SUPPLEMENTARY MATERIALS
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
-
FUNDING
The Vice-Chancellor of Research and Technology, Hamadan University of Medical Sciences funded this study (No. 9610266919).
-
AUTHOR CONTRIBUTIONS
Conceptualization: JP. Data curation: JP, LM, YM, ZC. Formal analysis: JP, LM. Funding acquisition: JP. Methodology: JP, LM, FGE. Writing – original draft: JP, ZC, YM. Writing – review & editing: LM, YM, ZC, FGE.
NOTES
ACKNOWLEDGEMENTS
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424.ArticlePubMed
- 2. Hisamichi S, Sasaki R, Sugawara N, Yanbo T, Yamagata S. Stomach cancer in various age groups (Japan) as detected by gastric mass survey. J Am Geriatr Soc 1979;27:439-443.ArticlePubMed
- 3. Dockerty JD, Marshall S, Fraser J, Pearce N. Stomach cancer in New Zealand: time trends, ethnic group differences and a cancer registry-based case-control study. Int J Epidemiol 1991;20:45-53.ArticlePubMedPDF
- 4. Sun H, Wu X, Wu F, Li Y, Yu Z, Chen X, et al. Associations of genetic variants in the PSCA, MUC1 and PLCE1 genes with stomach cancer susceptibility in a Chinese population. PLoS One 2015;10:e0117576.ArticlePubMedPMC
- 5. Mathew A, Gangadharan P, Varghese C, Nair MK. Diet and stomach cancer: a case-control study in South India. Eur J Cancer Prev 2000;9:89-97.ArticlePubMed
- 6. Praud D, Rota M, Pelucchi C, Bertuccio P, Rosso T, Galeone C, et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev 2018;27:124-133.ArticlePubMed
- 7. Minami Y, Kanemura S, Oikawa T, Suzuki S, Hasegawa Y, Miura K, et al. Associations of cigarette smoking and alcohol drinking with stomach cancer survival: a prospective patient cohort study in Japan. Int J Cancer 2018;143:1072-1085.ArticlePubMed
- 8. González CA, Sala N, Rokkas T. Gastric cancer: epidemiologic aspects. Helicobacter 2013;18 Suppl 1:34-38.ArticlePubMed
- 9. World Health Organization. Physical activity and young people; 2018 [cited 2018 Nov 12]. Available from: https://www.who.int/dietphysicalactivity/factsheet_young_people/en/.
- 10. Wells G, Shea B, O’connell DL, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; 2018 [cited 2020 Feb 10]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 11. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions; 2008 [cited 2020 Feb 10]. Available from: https://www.radioterapiaitalia.it/wp-content/uploads/2017/01/cochrane-handbook-for-systematic-reviews-of-interventions.pdf.
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560.ArticlePubMedPMC
- 13. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-634.ArticlePubMedPMC
- 14. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-1101.ArticlePubMed
- 15. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89-98.Article
- 16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.ArticlePubMed
- 17. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 2008;37:1148-1157.ArticlePubMedPMCPDF
- 18. Zhang Z, Zhang X. Salt taste preference, sodium intake and gastric cancer in China. Asian Pac J Cancer Prev 2011;12:1207-1210.PubMed
- 19. Zhong C, Li KN, Bi JW, Wang BC. Sodium intake, salt taste and gastric cancer risk according to Helicobacter pylori infection, smoking, histological type and tumor site in China. Asian Pac J Cancer Prev 2012;13:2481-2484.ArticlePubMedPDF
- 20. Szklo M, Nieto FJ. Epidemiology: beyond the basics. 4th. Burlington: Jones & Bartlett Learning; 2019. p 115.
- 21. Chiba T, Marusawa H, Seno H, Watanabe N. Mechanism for gastric cancer development by Helicobacter pylori infection. J Gastroenterol Hepatol 2008;23:1175-1181.ArticlePubMed
- 22. Hatakeyama M, Higashi H. Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci 2005;96:835-843.ArticlePubMed
- 23. World Health Organization. World No Tobacco Day 2017: beating tobacco for health, prosperity, the environment and national development; 2017 [cited 2017 Jun 1]. Available from: http://www.who.int/mediacentre/news/releases/2017/no-tobacco-day/en/.
- 24. Dyke GW, Craven JL, Hall R, Garner RC. Smoking-related DNA adducts in human gastric cancers. Int J Cancer 1992;52:847-850.ArticlePubMed
- 25. Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002;21:7435-7451.ArticlePubMedPDF
- 26. Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 2007;7:599-612.ArticlePubMedPDF
- 27. Deman J, Van Larebeke N. Carcinogenesis: mutations and mutagens. Tumour Biol 2001;22:191-202.ArticlePubMed
- 28. Huh K, Kwon TH, Shin US, Kim WB, Ahn BO, Oh TY, et al. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol 2003;88:269-273.ArticlePubMed
- 29. Kim HJ, Lim SY, Lee JS, Park S, Shin A, Choi BY, et al. Fresh and pickled vegetable consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci 2010;101:508-516.ArticlePubMed
- 30. Yalim S, Ozdemir Y. Effects of preparation procedures on ascorbic acid retention in pickled hot peppers. Int J Food Sci Nutr 2003;54:291-296.ArticlePubMed
- 31. Yang CS. Research on esophageal cancer in China: a review. Cancer Res 1980;40:2633-2644.PubMed
- 32. Akyön Y. Effect of antioxidants on the immune response of Helicobacter pylori. Clin Microbiol Infect 2002;8:438-441.ArticlePubMed
- 33. Drake IM, Davies MJ, Mapstone NP, Dixon MF, Schorah CJ, White KL, et al. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis 1996;17:559-562.ArticlePubMedPDF
- 34. Chen Y, Liu L, Wang X, Wang J, Yan Z, Cheng J, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev 2013;22:1395-1408.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

-
Gastric dysplasia in random biopsies: the influence of
Helicobacter pylori
infection and alcohol consumption in the presence of a lesion
Ana Isabel Ferreira, Tiago Lima Capela, Vítor Macedo Silva, Sofia Xavier, Pedro Boal Carvalho, Joana Magalhães, José Cotter
Scandinavian Journal of Gastroenterology.2024; 59(2): 125. CrossRef - Incidence of Stomach, Liver, and Colorectal Cancers by Geography and Social Vulnerability Among American Indian and Alaska Native Populations, 2010–2019
Stephanie C Melkonian, Melissa A Jim, Avid Reza, Lucy A Peipins, Donald Haverkamp, Nathania Said, J Danielle Sharpe
American Journal of Epidemiology.2024; 193(1): 58. CrossRef - Personalizing age of gastric cancer screening based on comorbidity in China: Model estimates of benefits, affordability and cost-effectiveness optimization
Shuxia Qin, Xuehong Wang, Sini Li, Meiyu Wu, Xiaomin Wan
Preventive Medicine.2024; 179: 107851. CrossRef - First evidence on the causal association between green tea and gastrointestinal health: a two sample mendelian randomization study
Zhixiong Jiang, Renlan Li, Yi Li
CyTA - Journal of Food.2024;[Epub] CrossRef - Dose–response association between cigarette smoking and gastric cancer risk: a systematic review and meta-analysis
Matteo Rota, Irene Possenti, Valeria Valsassina, Claudia Santucci, Vincenzo Bagnardi, Giovanni Corrao, Cristina Bosetti, Claudia Specchia, Silvano Gallus, Alessandra Lugo
Gastric Cancer.2024; 27(2): 197. CrossRef - Risk Factors of Gastric Cancer and Lifestyle Modification for Prevention
Kwang-Pil Ko
Journal of Gastric Cancer.2024; 24(1): 99. CrossRef - Uncovering the ceRNA Network Related to the Prognosis of Stomach Adenocarcinoma Among 898 Patient Samples
Zhe Liu, Fang Liu, Olutomilayo Olayemi Petinrin, Fuzhou Wang, Yu Zhang, Ka-Chun Wong
Biochemical Genetics.2024;[Epub] CrossRef - Pro-Inflammatory Diet as a Risk Factor for Stomach Cancer: Findings from a Multicenter Study in Central and Western China
Dan Li, Donglin Zhang, Minjuan Wang, Jianfeng Hao, Yongquan Shi, Dake Chu
Journal of Multidisciplinary Healthcare.2024; Volume 17: 901. CrossRef - A review of the world's salt reduction policies and strategies – preparing for the upcoming year 2025
Ting Nie, Siqi Huang, Yuxin Yang, Anna Hu, Jianing Wang, Zeneng Cheng, Wenjie Liu
Food & Function.2024; 15(6): 2836. CrossRef - Unraveling the causal role of immune cells in gastrointestinal tract cancers: insights from a Mendelian randomization study
Yu-xiang Wang, Chao-ping Zhou, Da-tian Wang, Jun Ma, Xue-hu Sun, Yao Wang, Ya-ming Zhang
Frontiers in Immunology.2024;[Epub] CrossRef - Targeting notch-related lncRNAs in cancer: Insights into molecular regulation and therapeutic potential
Raihan Siddique, Gaurav Gupta, Johar MGM, Ashwani Kumar, Harpreet Kaur, I.A. Ariffin, Atreyi Pramanik, Waleed Hassan Almalki, Haider Ali, Moyad Shahwan, Neeraj Patel, Krishna Murari, Riya Mishra, Riya Thapa, Asif Ahmad Bhat
Pathology - Research and Practice.2024; 257: 155282. CrossRef - Disulfidptosis-related lncRNA signature reveals immune microenvironment and novel molecular subtyping of stomach adenocarcinoma
Jinze Li, Chuqi Xia, Yilin Song, Lu Zhang, Wei Shang, Ning Xu, Qiyu Lu, Daoming Liang
Heliyon.2024; 10(8): e29005. CrossRef - Involvement of microRNA modifications in anticancer effects of major polyphenols from green tea, coffee, wine, and curry
Tomokazu Ohishi, Sumio Hayakawa, Noriyuki Miyoshi
Critical Reviews in Food Science and Nutrition.2023; 63(24): 7148. CrossRef - Characteristics of gastric cancer around the world
María J. López, Junior Carbajal, Alejandro L. Alfaro, Luis G. Saravia, Daniel Zanabria, Jhajaira M. Araujo, Lidia Quispe, Alejandra Zevallos, José L. Buleje, Cristina Eunbee Cho, Marisol Sarmiento, Joseph A. Pinto, Williams Fajardo
Critical Reviews in Oncology/Hematology.2023; 181: 103841. CrossRef - Association of Dietary Antioxidant Vitamin Intake and Gastric Cancer Risk According to Smoking Status and Histological Subtypes of Gastric Cancer: A Case-Control Study in Korea
Shin Ah Kim, Jung Hyun Kwak, Chang Soo Eun, Dong Soo Han, Yong Sung Kim, Kyu Sang Song, Bo Youl Choi, Hyun Ja Kim
Nutrition and Cancer.2023; 75(2): 652. CrossRef - Cancer and brassinosteroids: Mechanisms of action, SAR and future perspectives
Marcos Lorca, David Cabezas, Ileana Araque, Andrés Terán, Santiago Hernández, Marco Mellado, Luis Espinoza, Jaime Mella
Steroids.2023; 190: 109153. CrossRef - Risk factors for gastric cancer: A comprehensive analysis of observational studies
Yuqing Hui, Chunyi Tu, Danlei Liu, Huijie Zhang, Xiaobing Gong
Frontiers in Public Health.2023;[Epub] CrossRef - Smoking history and severe atrophic gastritis assessed by pepsinogen are risk factors for the prevalence of synchronous gastric cancers in patients with gastric endoscopic submucosal dissection: a multicenter prospective cohort study
Waku Hatta, Tomoyuki Koike, Sho Asonuma, Hideki Okata, Kaname Uno, Tomoyuki Oikawa, Wataru Iwai, Makoto Yonechi, Daisuke Fukushi, Shoichi Kayaba, Ryosuke Kikuchi, Motoki Ohyauchi, Jun Fushiya, Ryuhei Maejima, Yasuhiko Abe, Masashi Kawamura, Junya Honda, Y
Journal of Gastroenterology.2023; 58(5): 433. CrossRef - Association between soy products, fruits, vegetables, and dairy products and gastric cancer risk in Helicobacter pylori-infected subjects: a case-control study in Korea
Jung Hyun Kwak, Chang Soo Eun, Dong Soo Han, Yong Sung Kim, Kyu Sang Song, Bo Youl Choi, Hyun Ja Kim
Nutrition Research and Practice.2023; 17(1): 122. CrossRef - Evaluation of polygenic risk score for risk prediction of gastric cancer
Xiao-Yu Wang, Li-Li Wang, Lin Xu, Shu-Zhen Liang, Meng-Chao Yu, Qiu-Yue Zhang, Quan-Jiang Dong
World Journal of Gastrointestinal Oncology.2023; 15(2): 276. CrossRef - Mediating Role of Lifestyle Behaviors in the Association between Education and Cancer: Results from the European Prospective Investigation into Cancer and Nutrition
Alessandra Macciotta, Alberto Catalano, Maria Teresa Giraudo, Elisabete Weiderpass, Pietro Ferrari, Heinz Freisling, Sandra M. Colorado-Yohar, Carmen Santiuste, Pilar Amiano, Alicia K. Heath, Heather A. Ward, Sofia Christakoudi, Paolo Vineis, Deependra Si
Cancer Epidemiology, Biomarkers & Prevention.2023; 32(1): 132. CrossRef - Food Environment Index is Inversely Associated with Gastric Cancer Incidence in the United States
Shenghui Wu, Yanning Liu, Martie Thompson, Adam Hege
Nutrition and Cancer.2023; 75(4): 1123. CrossRef - Willingness to Undergo Gastroscopy for Early Gastric Cancer Screening and Its Associated Factors During the COVID-19 Pandemic – A Nationwide Cross-Sectional Study in China
Kejia Ma, Xuejie Chen, Xin Xiang, Xueyi Mao, Ningxin Zhu, Tianyu Wang, Shuyu Ye, Xiaoyan Wang, Minzi Deng
Patient Preference and Adherence.2023; Volume 17: 505. CrossRef - Constructing a novel mitochondrial-related gene signature for evaluating the tumor immune microenvironment and predicting survival in stomach adenocarcinoma
Jingjia Chang, Hao Wu, Jin Wu, Ming Liu, Wentao Zhang, Yanfen Hu, Xintong Zhang, Jing Xu, Li Li, Pengfei Yu, Jianjun Zhu
Journal of Translational Medicine.2023;[Epub] CrossRef - Clinical prognostic value of OSGIN2 in gastric cancer and its proliferative effect in vitro
Peipei Wang, Ying Zhu, Xinru Jia, Xiangchang Ying, Leitao Sun, Shanming Ruan
Scientific Reports.2023;[Epub] CrossRef - Yoghurt Intake and Gastric Cancer: A Pooled Analysis of 16 Studies of the StoP Consortium
Giulia Collatuzzo, Eva Negri, Claudio Pelucchi, Rossella Bonzi, Federica Turati, Charles S. Rabkin, Linda M. Liao, Rashmi Sinha, Domenico Palli, Monica Ferraroni, Lizbeth López-Carrillo, Nuno Lunet, Samantha Morais, Demetrius Albanes, Stephanie J. Weinste
Nutrients.2023; 15(8): 1877. CrossRef - Risk of cancer in patients with insomnia: Nationwide retrospective cohort study (2009–2018)
Kichul Yoon, Cheol Min Shin, Kyungdo Han, Jin Hyung Jung, Eun Hyo Jin, Joo Hyun Lim, Seung Joo Kang, Yoon Jin Choi, Dong Ho Lee, Dong Keon Yon
PLOS ONE.2023; 18(4): e0284494. CrossRef - Overview and countermeasures of cancer burden in China
Yian Wang, Qijia Yan, Chunmei Fan, Yongzhen Mo, Yumin Wang, Xiayu Li, Qianjin Liao, Can Guo, Guiyuan Li, Zhaoyang Zeng, Wei Xiong, He Huang
Science China Life Sciences.2023; 66(11): 2515. CrossRef - The Influence of Helicobacter pylori on Human Gastric and Gut Microbiota
Marcello Fiorani, Ege Tohumcu, Livio Enrico Del Vecchio, Serena Porcari, Giovanni Cammarota, Antonio Gasbarrini, Gianluca Ianiro
Antibiotics.2023; 12(4): 765. CrossRef - Spatial and temporal analysis of gastric cancer incidence in northwest Iran
Mohsen Soleimani, Mohammad Reza Saeini, Ahmad Jalilvand
GeoJournal.2023; 88(4): 4555. CrossRef - The prevalence and determinant of overweight and obesity among residents aged 40–69 years in high-risk regions for upper gastrointestinal cancer in southeast China
Xiang Feng, Jinhua Zhu, Zhaolai Hua, Qiuping Shi, Jinyi Zhou, Pengfei Luo
Scientific Reports.2023;[Epub] CrossRef - BASP1 expression is associated with poor prognosis and is correlated with immune infiltration in gastric cancer
Tao Wang, Xiaojing Liu, Tong Wang, Lei Zhan, Mingjun Zhang
FEBS Open Bio.2023; 13(8): 1507. CrossRef - Cancer incidence in Southern Iran, 2015−2018: A population based study on cancer registry profile of Fars province
Abbas Rezaianzadeh, Masoumeh Ghoddusi Johari, Hamid Reza Niazkar, Zahra Khosravizadegan, Ahmad Monabati, Babak Shiraziyeganeh
Health Science Reports.2023;[Epub] CrossRef - Bioinformatics Analysis Reveals the Vital Role of AKR1B1 in Immune Infiltration and Clinical Outcomes of Gastric Cancer
Zhiyue Zhao, Zhibin Hao, Zheng Zhang, Xianbao Zhan
DNA and Cell Biology.2023; 42(7): 372. CrossRef - Risk factor profiles for gastric cancer prediction with respect to Helicobacter pylori: A study of a tertiary care hospital in Pakistan
Shahid Aziz, Simone König, Muhammad Umer, Tayyab Saeed Akhter, Shafqat Iqbal, Maryum Ibrar, Tofeeq Ur-Rehman, Tanvir Ahmad, Alfizah Hanafiah, Rabaab Zahra, Faisal Rasheed
Artificial Intelligence in Gastroenterology.2023; 4(1): 10. CrossRef - Functional activity of the monocyte immune link in gastric adenocarcinoma
O. V. Smirnova, E. S. Ovcharenko
Medical Immunology (Russia).2023; 25(5): 1117. CrossRef - Clinical supervision of chronic atrophic gastritis
M. A. Livzan, O. V. Gaus, M. A. Lisovskiy, S. I. Mozgovoi, V. A. Rubtsov, M. N. Parygina
Experimental and Clinical Gastroenterology.2023; (3): 148. CrossRef - Critical Analysis of Risk Factors and Machine-Learning-Based Gastric Cancer Risk Prediction Models: A Systematic Review
Zeyu Fan, Ziju He, Wenjun Miao, Rongrong Huang
Processes.2023; 11(8): 2324. CrossRef - Helicobacter pylori infection altered gastric microbiota in patients with chronic gastritis
Zhaolai Hua, Le Xu, Jiahui Zhu, Ling Xiao, Bin Lu, Jianping Wu, Zhenfeng Wu, Qihai Zhou, Junfeng Zhang
Frontiers in Cellular and Infection Microbiology.2023;[Epub] CrossRef - The influence of obesity on the risk of development of selected gastrointestinal cancers
Anita Marcinkiewicz, Karolina Borowska-Waniak, Aneta Łukaszczyk, Aleksandra Ochotnicka, Anna Opala, Maja Borowska, Kinga Skorupińska, Dominik Michalik
Humanities & Social Sciences Reviews.2023; 11(4): 44. CrossRef - Extension of resection after positive intraoperative pathology during surgery for gastric and gastroesophageal junction adenocarcinoma: a retrospective cohort study
Patrick S. Plum, Atakan G. Barutcu, Aylin Pamuk, Christoph Mallmann, Seung-Hun Chon, Costanza Chiapponi, Martin Dübbers, Martin Hellmich, Stefan P. Moenig, Alexander Quaas, Arnulf H. Hoelscher, Christiane J. Bruns, Hakan Alakus
International Journal of Surgery.2023; 109(8): 2324. CrossRef - Vegetarian diets and the risk of gastrointestinal cancers: a meta-analysis of observational studies
Tongtong Bai, Juanjuan Peng, Xinqi Zhu, Chengyu Wu
European Journal of Gastroenterology & Hepatology.2023; 35(11): 1244. CrossRef - Lifestyle habits and gastric cancer in an East Asian population: a Mendelian randomization study
Yuegui Tan, Zhao Wei, Kun Liu, Yuzhen Qin, Wenqi Hui
Frontiers in Oncology.2023;[Epub] CrossRef - Complete mesogastric excision for gastric cancer: is it the future of gastric cancer surgery?
Georgios D Lianos, Christina D Bali, Konstantinos Vlachos, Panagiota Drosou, Stefano Rausei, Michail Mitsis, Dimitrios Schizas
Personalized Medicine.2023; 20(5): 461. CrossRef - Toxic mechanisms of cadmium and exposure as a risk factor for oral and gastrointestinal carcinomas
Ali Tavakoli Pirzaman, Pouyan Ebrahimi, Shokat Niknezhad, Turan vahidi, Dariush Hosseinzadeh, Sousan Akrami, Arash M Ashrafi, Mohammad Moeen Velayatimehr, Rezvan Hosseinzadeh, Sohrab Kazemi
Human & Experimental Toxicology.2023;[Epub] CrossRef - Role of microorganisms in the developmental, prognostic, and therapeutics of gastric cancer
Sadaf Wajahat
Iranian Journal of Blood and Cancer.2023; 15(4): 272. CrossRef - Association of Mediterranean Diet Adherence with Disease Progression Characteristics, Lifestyle Factors and Overall Survival in Gastric Cancer Patients
Eleni Pavlidou, Sousana K. Papadopoulou, Maria Tolia, Maria Mentzelou, Nikolaos Tsoukalas, Olga Alexatou, Theodora Tsiouda, Gerasimos Tsourouflis, Evmorfia Psara, Vasileios Bikos, Nikolaos Kavantzas, Ioly Kotta-Loizou, Antonios Dakanalis, Theofanis Vorvol
Medical Sciences.2023; 11(4): 74. CrossRef - Nutrigenomics and microbiome shaping the future of personalized medicine: a review article
Neemat M. Kassem, Yassmin A. Abdelmegid, Mahmoud K. El-Sayed, Rana S. Sayed, Mahmoud H. Abdel-Aalla, Hebatallah A. Kassem
Journal of Genetic Engineering and Biotechnology.2023; 21(1): 134. CrossRef - EXPLORATORY ANALYSIS OF DIETARY PATTERNS OF PATIENTS WITH GASTRIC ADENOCARCINOMA: A CASE-CONTROL STUDY IN CENTRAL BRAZIL
Silvana Barbosa SANTIAGO, Gabriela Rodrigues de SOUSA, Amanda Ferreira Paes Landim RAMOS, Gisele Aparecida FERNANDES, Maria Paula CURADO, Mônica Santiago BARBOSA
Arquivos de Gastroenterologia.2023; 60(4): 419. CrossRef - Understanding knowledge, attitudes and behaviours related to dietary sodium intake in a multi-ethnic population in Singapore
Cindy Mei Jun Chan, Borame Sue Lee Dickens, Mary Foong-Fong Chong
Public Health Nutrition.2023; 26(12): 2802. CrossRef - Emerging Trends in Metal‐based Anticancer Agents: Drug Design to Clinical Trials and their Mechanism of Action
Achamo Temesgen, Hanabe Chowdappa Ananda Murthy, Amare Zereffa Enyew, Rajappan Revathi, Ramachandran Venkatesha Perumal
ChemistrySelect.2023;[Epub] CrossRef - Consumption of Fruits and Vegetables and Gastric Cancer Risk: Answers From Case-Control Study
Maryam Aljumaily, Noora Al-Naimi, Rawdhah Al-Amer, Aya Hamdan, Sabika Allehdan, Tareq Al-Jaberi, Ahmad Hushki, Yaser Rayyan, Reema Tayyem
American Journal of Lifestyle Medicine.2023;[Epub] CrossRef - Decreased expression of TRIM3 gene predicts a poor prognosis in gastric cancer
Javad Farhadi, Ladan Goshayeshi, Alireza Motavalizadehkakhky, Jamshid Mehrzad, Hassan Mehrad-Majd
Journal of Gastrointestinal Cancer.2022; 53(1): 179. CrossRef - Association between the Persistence of Obesity and the Risk of Gastric Cancer: A Nationwide Population-Based Study
Joo Hyun Lim, Cheol Min Shin, Kyung-Do Han, Seung Woo Lee, Eun Hyo Jin, Yoon Jin Choi, Hyuk Yoon, Young Soo Park, Nayoung Kim, Dong Ho Lee
Cancer Research and Treatment.2022; 54(1): 199. CrossRef - Decisional balance, self-leadership, self-efficacy, planning, and stages of change in adopting exercise behaviors in patients with stomach cancer: A cross-sectional study
Myung Kyung Lee
European Journal of Oncology Nursing.2022; 56: 102086. CrossRef - Epidemiology of Helicobacter pylori
Amnon Sonnenberg
Alimentary Pharmacology & Therapeutics.2022;[Epub] CrossRef - Functional polysaccharide lentinan: Role in anti-cancer therapies and management of carcinomas
Sagar Trivedi, Krishna Patel, Veena Belgamwar, Kamlesh Wadher
Pharmacological Research - Modern Chinese Medicine.2022; 2: 100045. CrossRef - Ultrasound Image-Guided Nerve Block Combined with General Anesthesia under an Artificial Intelligence Algorithm on Patients Undergoing Radical Gastrectomy for Gastric Cancer during and after Operation
Wanqiu Fan, Liuyingzi Yang, Jing Li, Biqian Dong, Osamah Ibrahim Khalaf
Computational and Mathematical Methods in Medicine.2022; 2022: 1. CrossRef - The impact of excessive salt intake on human health
Robert W. Hunter, Neeraj Dhaun, Matthew A. Bailey
Nature Reviews Nephrology.2022; 18(5): 321. CrossRef - Gastric epithelial histology and precancerous conditions
Hang Yang, Wen-Juan Yang, Bing Hu
World Journal of Gastrointestinal Oncology.2022; 14(2): 396. CrossRef - ELP6 and PLIN5 Mutations Were Probably Prognostic Biomarkers for Patients With Gastric Cancer
Ji Di, Yan Chai, Xin Yang, Haibin Dong, Bo Jiang, Faxiang Ji
Frontiers in Medicine.2022;[Epub] CrossRef - H. pylori Infection and Virulence Factors cagA and vacA (s and m Regions) in Gastric Adenocarcinoma from Pará State, Brazil
Igor Brasil-Costa, Cintya de Oliveira Souza, Leni Célia Reis Monteiro, Maria Elisabete Silva Santos, Edivaldo Herculano Correa De Oliveira, Rommel Mario Rodriguez Burbano
Pathogens.2022; 11(4): 414. CrossRef - Investigating the Prognostic Significance of Pyroptosis-Related Genes in Gastric Cancer and Their Impact on Cells’ Biological Functions
Jie Yin, Gang Che, Wankun Wang, Shitu Chen, Jian Liu
Frontiers in Oncology.2022;[Epub] CrossRef - Epidemiology of stomach cancer
Milena Ilic, Irena Ilic
World Journal of Gastroenterology.2022; 28(12): 1187. CrossRef - Effect of Apatinib Combined with Seggio on the Expression of Serum AFP and CA724 and Long-Term Survival Rate in Patients with Advanced Gastric Cancer Undergoing Comfortable Nursing Intervention
Dawei Ren, Mi Feng, Shengmin Zhang, Yun Zhang, Ji Li, Suneet Kumar Gupta
Journal of Healthcare Engineering.2022; 2022: 1. CrossRef - Parent Fruit and Vegetable Consumption Outcomes from the Translational ‘Time for Healthy Habits’ Trial: Secondary Outcomes from a Partially Randomized Preference Trial
Rebecca J. Wyse, Jacklyn K. Jackson, Megan L. Hammersley, Fiona Stacey, Rachel A. Jones, Anthony Okely, Amanda Green, Sze Lin Yoong, Christophe Lecathelinais, Christine Innes-Hughes, Joe Xu, Karen Gillham, Chris Rissel
International Journal of Environmental Research and Public Health.2022; 19(10): 6165. CrossRef - Tea consumption and gastric cancer: a pooled analysis from the Stomach cancer Pooling (StoP) Project consortium
Georgia Martimianaki, Gianfranco Alicandro, Claudio Pelucchi, Rossella Bonzi, Matteo Rota, Jinfu Hu, Kenneth C. Johnson, Charles S. Rabkin, Linda M. Liao, Rashmi Sinha, Zuo-Feng Zhang, Michela Dalmartello, Nuno Lunet, Samantha Morais, Domenico Palli, Moni
British Journal of Cancer.2022; 127(4): 726. CrossRef - Sex and gender disparities in patients with advanced gastroesophageal adenocarcinoma: data from the AGAMENON-SEOM registry
J. Gallego Plazas, A. Arias-Martinez, A. Lecumberri, E. Martínez de Castro, A. Custodio, J.M. Cano, R. Hernandez, A.F. Montes, I. Macias, A. Pieras-Lopez, M. Diez, L. Visa, R.V. Tocino, N. Martínez Lago, M.L. Limón, M. Gil, P. Pimentel, M. Mangas, M. Gran
ESMO Open.2022; 7(3): 100514. CrossRef - Vitamin D—The Nutritional Status of Post-Gastrectomy Gastric Cancer Patients—Systematic Review
Tomasz Muszyński, Karina Polak, Aleksandra Frątczak, Bartosz Miziołek, Beata Bergler-Czop, Antoni Szczepanik
Nutrients.2022; 14(13): 2712. CrossRef - Serum Pepsinogen as a Biomarker for Gastric Cancer in the United States: A Nested Case–Control Study Using the PLCO Cancer Screening Trial Data
Haejin In, Srawani Sarkar, Jessica Ward, Patricia Friedmann, Michael Parides, Julie Yang, Meira Epplein
Cancer Epidemiology, Biomarkers & Prevention.2022; 31(7): 1426. CrossRef - A prospective cohort study on the association between waterpipe tobacco smoking and gastric cancer mortality in Northern Vietnam
Hung Xuan Le, Dung Thi Thuy Truong, Long Bao Tran, Phuoc Hong Le, Binh Uyen Duong Pham, Koji Wada, Shunya Ikeda, Ariuntuul Garidkhuu, Can Van Phan, Ngoan Tran Le
BMC Cancer.2022;[Epub] CrossRef - Microbiota and the Immune System—Actors in the Gastric Cancer Story
Marek Majewski, Paulina Mertowska, Sebastian Mertowski, Konrad Smolak, Ewelina Grywalska, Kamil Torres
Cancers.2022; 14(15): 3832. CrossRef - Benefit-to-harm ratio and cost-effectiveness of government-recommended gastric cancer screening in China: A modeling study
Shuxia Qin, Xuehong Wang, Sini Li, Chongqing Tan, Xiaohui Zeng, Meiyu Wu, Ye Peng, Liting Wang, Xiaomin Wan
Frontiers in Public Health.2022;[Epub] CrossRef - Machine learning: A non-invasive prediction method for gastric cancer based on a survey of lifestyle behaviors
Siqing Jiang, Haojun Gao, Jiajin He, Jiaqi Shi, Yuling Tong, Jian Wu
Frontiers in Artificial Intelligence.2022;[Epub] CrossRef - Effects of the Wnt/β-Catenin Signaling Pathway on Proliferation and Apoptosis of Gastric Cancer Cells
Jia Chen, Xingyu Wang, Jianlin Zhang, Jiawei Chang, Chuanjun Han, Zhouwei Xu, Hongzhu Yu, Yuvaraja Teekaraman
Contrast Media & Molecular Imaging.2022; 2022: 1. CrossRef - Microbial Proteins in Stomach Biopsies Associated with Gastritis, Ulcer, and Gastric Cancer
Shahid Aziz, Faisal Rasheed, Tayyab Saeed Akhter, Rabaab Zahra, Simone König
Molecules.2022; 27(17): 5410. CrossRef - The role of bariatric and metabolic surgery in the development, diagnosis, and treatment of endometrial cancer
Robert C. Ross, Yetunde M. Akinde, Philip R. Schauer, Carel W. le Roux, Donal Brennan, Amelia M. Jernigan, Marco Bueter, Vance L. Albaugh
Frontiers in Surgery.2022;[Epub] CrossRef - A prognostic signature of pyroptosis-related lncRNAs verified in gastric cancer samples to predict the immunotherapy and chemotherapy drug sensitivity
Yanan Wang, Xiaowei Chen, Fei Jiang, Yan Shen, Fujin Fang, Qiong Li, Chuanli Yang, Yu Dong, Xiaobing Shen
Frontiers in Genetics.2022;[Epub] CrossRef - Prediction of gastric cancer risk by a polygenic risk score of Helicobacter pylori
Xiao-Yu Wang, Li-Li Wang, Shu-Zhen Liang, Chao Yang, Lin Xu, Meng-Chao Yu, Yi-Xuan Wang, Quan-Jiang Dong
World Journal of Gastrointestinal Oncology.2022; 14(9): 1844. CrossRef - The origin of gastric cancer stem cells and their effects on gastric cancer: Novel therapeutic targets for gastric cancer
Ying Yang, Wen-Jian Meng, Zi-Qiang Wang
Frontiers in Oncology.2022;[Epub] CrossRef - Analysis of the Preventive Action of Rivaroxaban against Lower Extremity Deep Venous Thrombosis in Patients after Laparoscopic Radical Gastrectomy
Qinhui Dong, Xiayin Zhu, Yafen Gao, Zhengrong Wang, Dexing Zheng, Jian Zhu, Pan Zheng
Computational and Mathematical Methods in Medicine.2022; 2022: 1. CrossRef - LASTR is a novel prognostic biomarker and predicts response to cancer immunotherapy in gastric cancer
Jun-Yan Liu, Jing Yao, Jia-Jia Liu, Tao He, Fang-Jie Wang, Tian-Yu Xie, Jian-Xin Cui, Xiao-Dong Yang
Frontiers in Oncology.2022;[Epub] CrossRef - Diet and carcinogenesis of gastric cancer
Gautam Maddineni, Jesse J. Xie, Bhaumik Brahmbhatt, Pritesh Mutha
Current Opinion in Gastroenterology.2022; 38(6): 588. CrossRef - The stomach cancer prognosis map is the basis for the formation of a register of patients with precancerous diseases
A. Yu. Baranovsky, T. L. Tsvetkova
Experimental and Clinical Gastroenterology.2022; (9): 39. CrossRef - A Survey on the Actual Use of and Reasons for Heated Tobacco Products in Patients with Rheumatoid Arthritis
Hisaaki Isaji, Kiyofumi Yamada
International Journal of Environmental Research and Public Health.2022; 19(19): 12465. CrossRef - Prevalence of Her2-neu status and its clinicopathological association in newly diagnosed gastric cancer patients
Joseph Kattan, Fady el Karak, Fadi Farhat, Dany Abi Gerges, Walid Mokaddem, Georges Chahine, Saad Khairallah, Najla Fakhruddin, Jawad Makarem, Fadi Nasr
BMC Cancer.2022;[Epub] CrossRef - Topical issues of prevention of stomach cancer: A review
Yury P. Uspenskiy, Natalia V. Baryshnikova, Alexey A. Krasnov, Sergey V. Petlenko, Vera A. Apryatina
Consilium Medicum.2022; 24(5): 358. CrossRef - Helicobacter pylori-Positive Gastric Biopsies—Association with Clinical Predictors
Anca Negovan, Andreea-Raluca Szőke, Simona Mocan, Claudia Bănescu
Life.2022; 12(11): 1789. CrossRef - Meat Intake, Cooking Methods, Doneness Preferences and Risk of Gastric Adenocarcinoma in the MCC-Spain Study
Elena Boldo, Nerea Fernández de Larrea, Marina Pollán, Vicente Martín, Mireia Obón-Santacana, Marcela Guevara, Gemma Castaño-Vinyals, Jose María Canga, Beatriz Pérez-Gómez, Inés Gómez-Acebo, Guillermo Fernández-Tardón, Mercedes Vanaclocha-Espi, Rocío Olme
Nutrients.2022; 14(22): 4852. CrossRef - The Regulatory Network of Gastric Cancer Pathogenesis and Its Potential Therapeutic Active Ingredients of Traditional Chinese Medicine Based on Bioinformatics, Molecular Docking, and Molecular Dynamics Simulation
Peng Yang, Peng Liu, Junmao Li, Mohammad Jahoor Alam
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - IL-17 Receptor Signaling through IL-17A or IL-17F Is Sufficient to Maintain Innate Response and Control of Helicobacter pylori Immunopathogenesis
Beverly R. E. A. Dixon, Tiffany J. Lee, Diana C. Contreras Healey, Jing Li, Jeremy A. Goettel, M. Blanca Piazuelo, Holly M. Scott Algood
ImmunoHorizons.2022; 6(2): 116. CrossRef - Gastric cancer risk is reduced by a predominance of antioxidant factors in the oxidative balance: a hospital-based case-control study in Korea
Jimi Kim, Jeonghee Lee, Il Ju Choi, Young-Il Kim, Jeongseon Kim
Epidemiology and Health.2022; 44: e2022089. CrossRef - Coffee consumption and gastric cancer: a pooled analysis from the Stomach cancer Pooling Project consortium
Georgia Martimianaki, Paola Bertuccio, Gianfranco Alicandro, Claudio Pelucchi, Francesca Bravi, Greta Carioli, Rossella Bonzi, Charles S. Rabkin, Linda M. Liao, Rashmi Sinha, Ken Johnson, Jinfu Hu, Domenico Palli, Monica Ferraroni, Nuno Lunet, Samantha Mo
European Journal of Cancer Prevention.2022; 31(2): 117. CrossRef - Hydrogen inhibits the proliferation and migration of gastric cancer cells by modulating lncRNA MALAT1/miR-124-3p/EZH2 axis
Baocheng Zhu, Hengguan Cui, Weiqiang Xu
Cancer Cell International.2021;[Epub] CrossRef - Diet and cancer of the esophagus and stomach
Shu Wen Tay, James Weiquan Li, Kwong Ming Fock
Current Opinion in Gastroenterology.2021; 37(2): 158. CrossRef - Gastric Cancer Risk Prediction Using an Epidemiological Risk Assessment Model and Polygenic Risk Score
Boyoung Park, Sarah Yang, Jeonghee Lee, Il Ju Choi, Young-Il Kim, Jeongseon Kim
Cancers.2021; 13(4): 876. CrossRef - Transcriptome Analysis of Subcutaneous Adipose Tissue from Severely Obese Patients Highlights Deregulation Profiles in Coding and Non-Coding Oncogenes
Federica Rey, Letizia Messa, Cecilia Pandini, Rossella Launi, Bianca Barzaghini, Giancarlo Micheletto, Manuela Teresa Raimondi, Simona Bertoli, Cristina Cereda, Gian Vincenzo Zuccotti, Raffaella Cancello, Stephana Carelli
International Journal of Molecular Sciences.2021; 22(4): 1989. CrossRef - Risk of gastric cancer in the environs of industrial facilities in the MCC-Spain study
Javier García-Pérez, Virginia Lope, Nerea Fernández de Larrea-Baz, Antonio J. Molina, Adonina Tardón, Juan Alguacil, Beatriz Pérez-Gómez, Víctor Moreno, Marcela Guevara, Gemma Castaño-Vinyals, José J. Jiménez-Moleón, Inés Gómez-Acebo, Ana Molina-Barceló,
Environmental Pollution.2021; 278: 116854. CrossRef - Factors Related to Lacking Knowledge on the Recommended Daily Salt Intake among Medical Professionals in Mongolia: A Cross-Sectional Study
Naoko Hikita, Enkhtungalag Batsaikhan, Satoshi Sasaki, Megumi Haruna, Ariunaa Yura, Otgontogoo Oidovsuren
International Journal of Environmental Research and Public Health.2021; 18(8): 3850. CrossRef - A Physically Active Status Affects the Circulating Profile of Cancer-Associated miRNAs
Martina Faraldi, Laura Gerosa, Marta Gomarasca, Veronica Sansoni, Silvia Perego, Ewa Ziemann, Giuseppe Banfi, Giovanni Lombardi
Diagnostics.2021; 11(5): 820. CrossRef - Association between obesity and the risk of gastric cancer in premenopausal and postmenopausal women: A nationwide cohort study
In Young Choi, Yoon Jin Choi, Dong Wook Shin, Kyung Do Han, Keun Hye Jeon, Su‐Min Jeong, Jung Eun Yoo
Journal of Gastroenterology and Hepatology.2021; 36(10): 2834. CrossRef - Dose–Effect Relationship Between Gastric Cancer and Common Digestive Tract Symptoms and Diagnoses in Anhui, China
Mengsha Tang, Xingrong Shen, Jing Chai, Jing Cheng, Debin Wang
Cancer Management and Research.2021; Volume 13: 4955. CrossRef - Metaplot: A new Stata module for assessing heterogeneity in a meta-analysis
Jalal Poorolajal, Shahla Noornejad, Mohammad Asghari Jafarabadi
PLOS ONE.2021; 16(6): e0253341. CrossRef - NF-κB in Gastric Cancer Development and Therapy
Supattra Chaithongyot, Phatcharida Jantaree, Olga Sokolova, Michael Naumann
Biomedicines.2021; 9(8): 870. CrossRef - Does coffee, tea and caffeine consumption reduce the risk of incident breast cancer? A systematic review and network meta-analysis
Shu Wang, Xiang Li, Yue Yang, Jingping Xie, Mingyue Liu, Ya Zhang, Yingshi Zhang, Qingchun Zhao
Public Health Nutrition.2021; 24(18): 6377. CrossRef - Risk factors for gastric cancer: a large-scale, population-based case-control study
Rui Zhang, He Li, Ni Li, Ju-Fang Shi, Jiang Li, Hong-Da Chen, Yi-Wen Yu, Chao Qin, Jian-Song Ren, Wan-Qing Chen, Jie He
Chinese Medical Journal.2021; 134(16): 1952. CrossRef - Gastric cancer mortality related to direct radiographic and endoscopic screening: A retrospective study
Hiroaki Hagiwara, Fumitaka Moki, Yukiko Yamashita, Kazuki Saji, Keigo Iesaki, Hiromitsu Suda
World Journal of Gastroenterology.2021; 27(33): 5595. CrossRef - Factores de riesgo para cáncer gástrico: ¿cuál es su papel?
Ricardo Oliveros Wilches, Helena Facundo Navia, Ana Deise Bonilla Castañeda, Raúl Eduardo Pinilla Morales
Revista colombiana de Gastroenterología.2021; 36(3): 366. CrossRef - Development and External Validation of a Nomogram for Predicting Overall Survival in Stomach Cancer: A Population-Based Study
Haonan Ji, Huita Wu, Yu Du, Li Xiao, Yiqin Zhang, Qiuhua Zhang, Xin Wang, Wenfeng Wang, Nahrizul Adib Kadri
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef - Fruit consumption and multiple health outcomes: An umbrella review
Liuqiao Sun, Xiaoping Liang, Yaoyao Wang, Sui Zhu, Qian Ou, Hang Xu, Fangyuan Li, Xuying Tan, Zhiwei Lai, Liuzhen Pu, Xingyi Chen, Jun Wei, Feng Wu, Huilian Zhu, Lijun Wang
Trends in Food Science & Technology.2021; 118: 505. CrossRef - Testing and Treating Helicobacter pylori Infection in Individuals With Family History of Gastric Cancer is Cost-effective
Sheila D. Rustgi, Aaron Oh, Chin Hur
Gastroenterology.2021; 161(6): 2051. CrossRef - Factors for the Primary Prevention of Breast Cancer: A Meta-Analysis of Prospective Cohort Studies
Jalal Poorolajal, Fatemeh Heidarimoghis, Manoochehr Karami, Zahra Cheraghi, Fatemeh Gohari-Ensaf, Fatemeh Shahbazi, Bushra Zareie, Pegah Ameri, Fatemeh Sahraei
Journal of Research in Health Sciences.2021; 21(3): e00520. CrossRef - Risk Prediction for Gastric Cancer Using GWAS-Identifie Polymorphisms, Helicobacter pylori Infection and Lifestyle-Related Risk Factors in a Japanese Population
Naoyo Ishikura, Hidemi Ito, Isao Oze, Yuriko N. Koyanagi, Yumiko Kasugai, Yukari Taniyama, Yukino Kawakatsu, Tsutomu Tanaka, Seiji Ito, Masahiro Tajika, Yasuhiro Shimizu, Yasumasa Niwa, Keitaro Matsuo
Cancers.2021; 13(21): 5525. CrossRef - Long noncoding RNA NR2F1-AS1 plays a carcinogenic role in gastric cancer by recruiting transcriptional factor SPI1 to upregulate ST8SIA1 expression
Fang Zuo, Yong Zhang, Jianting Li, Shaoxiang Yang, Xiaolu Chen
Bioengineered.2021; 12(2): 12345. CrossRef - Histórico familiar de câncer gástrico em pacientes dispépticos indicados à triagem endoscópica
Maria Carolina Pereira Rodrigues, Victor Pereira Lima, Flavia Ferreira Monari, Roberta de Araújo e Silva, Liana Mara Rocha Teles, Eveline Pinheiro Beserra, Maria Alzete de Lima, Maria Aparecida Alves de Oliveira Serra
Acta Paulista de Enfermagem.2021;[Epub] CrossRef - Contributing factors common to COVID‑19 and gastrointestinal cancer
Ronald Kostoff, Michael Briggs, Darja Kanduc, Darla Shores, Leda Kovatsi, Nikolaos Drakoulis, Alan Porter, Aristidis Tsatsakis, Demetrios Spandidos
Oncology Reports.2021;[Epub] CrossRef - Perfil de citocinas Th1, Th2, Th17 y otras citocinas pro inflamatorias (IL-1β, IL-6 y TNFα) en el plasma de pacientes con cáncer gástrico
Carmen Villagran, Rafael Fernández-Botrán, Elisa Hernandez, Federico Nave, Irmgardt A. Wellmann, Jose F. Muñoz-Valle
Ciencia, Tecnología y Salud.2021; 8(2): 166. CrossRef - SOLUBLE FORMS OF PD-1 AND PD-L1 IN BLOOD PLASMA OF GASTRIC CANCER PATIENTS AND THEIR ASSOCIATIONS WITH CLINICAL AND MORPHOLOGICAL CHARACTERISTICS OF THE DISEASE
E. S. Gershtein, N. A. Ognerubov, V. L. Chang, V. V. Delektorskaya, E. A. Korotkova, N. Yu. Sokolov, S. B. Polikarpova, I. S. Stilidi, Nikolay Evgenievich Kushlinskii
Russian Clinical Laboratory Diagnostics.2020; 65(6): 347. CrossRef - Gastric carcinoma: Insights into risk factors, methods of diagnosis, possible lines of management, and the role of primary care
AliyahM Marghalani, ThekraO Bin Salman, FawazJ Faqeeh, MohammedK Asiri, AhmedM Kabel
Journal of Family Medicine and Primary Care.2020; 9(6): 2659. CrossRef - Progress in cancer mortality, incidence, and survival: a global overview
Claudia Santucci, Greta Carioli, Paola Bertuccio, Matteo Malvezzi, Ugo Pastorino, Paolo Boffetta, Eva Negri, Cristina Bosetti, Carlo La Vecchia
European Journal of Cancer Prevention.2020; 29(5): 367. CrossRef - Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals
James W. T. Toh, Robert B. Wilson
International Journal of Molecular Sciences.2020; 21(17): 6451. CrossRef - Alterations in Gastric Microbial Communities Are Associated with Risk of Gastric Cancer in a Korean Population: A Case-Control Study
Madhawa Gunathilake, Jeonghee Lee, Il Ju Choi, Young-Il Kim, Jaekyung Yoon, Woo Jun Sul, Jihyun F. Kim, Jeongseon Kim
Cancers.2020; 12(9): 2619. CrossRef - Identification of Key Genes in Gastric Cancer by Bioinformatics Analysis
Xinyu Chong, Rui Peng, Yan Sun, Luyu Zhang, Zheng Zhang
BioMed Research International.2020; 2020: 1. CrossRef - Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid
Sumio Hayakawa, Tomokazu Ohishi, Noriyuki Miyoshi, Yumiko Oishi, Yoriyuki Nakamura, Mamoru Isemura
Molecules.2020; 25(19): 4553. CrossRef - Bioinformatics Analysis Reveals an Association Between Cancer Cell Stemness, Gene Mutations, and the Immune Microenvironment in Stomach Adenocarcinoma
Zaisheng Ye, Miao Zheng, Yi Zeng, Shenghong Wei, Yi Wang, Zhitao Lin, Chen Shu, Yunqing Xie, Qiuhong Zheng, Luchuan Chen
Frontiers in Genetics.2020;[Epub] CrossRef
- Figure
- Related articles
-
- Effectiveness of community-based interventions for older adults living alone: a systematic review and meta-analysis
- Dietary intake and cancer incidence in Korean adults: a systematic review and meta-analysis of observational studies
- Cancer risk based on alcohol consumption levels: a comprehensive systematic review and meta-analysis
- The prevalence of Q fever in the Eastern Mediterranean region: a systematic review and meta-analysis
- The prevalence of functional disability and its impact on older adults in the ASEAN region: a systematic review and meta-analysis

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite